By Stephen O’Quinn, Vice President of Medical Affairs, Zynerba Pharmaceuticals
Hello to everyone who will be attending the 18th International Fragile X Conference and the entire Fragile X community.
On behalf of the entire team at Zynerba Pharmaceuticals, we are very happy and honored to attend the meeting with you and to support the National Fragile X Foundation and the Fragile X community.
To all of the individuals living with Fragile X and their families and caregivers, to the clinicians and staff who help manage and treat Fragile X, we appreciate your remarkable support of the Fragile X community. Your efforts inspire us all.
Zynerba is committed to achieving our goal, with your help, of successfully developing a new medicine to treat the behavioral symptoms of Fragile X that, if approved, may improve the lives of children and their families living with Fragile X.
The next step in achieving our goal is to complete enrollment in the ongoing RECONNECT clinical trial.
You can learn more about the trial by visiting the RECONNECT booth (#14) at the conference in San Diego where we will have a team of individuals to discuss the trial with you.
Don’t worry if you can’t attend in person. You may also learn more about the trial by:
- Visiting our virtual RECONNECT booth in the conference meeting app (register here↗)
- Visiting the RECONNECT Fragile X Study Website↗
- Reviewing the RECONNECT posting on the MyFXResearch portal
You may apply in person at the meeting or online for your child to participate. Either way, you will have the opportunity to discuss whether your child may be eligible to participate and, if so, be referred to a clinical site closest to your home. Clinical sites are located in the U.S., Australia, U.K., and Ireland.
We are dedicated to supporting this incredible community and we are excited to see everyone at the conference in July.
author

Stephen O’Quinn, PharmD
Dr. O’Quinn is a senior pharmaceutical executive with over 30 years of experience in clinical development, medical affairs, and commercialization of medicines in multiple therapy areas, including neurology and psychiatry. Prior to joining Zynerba, Dr. O’Quinn spent over 20 years with GlaxoSmithKline in senior leadership roles. Following his time at GlaxoSmithKline, he spent five years as a consultant to pharmaceutical companies supporting clinical development and medical affairs activities. Dr. O’Quinn earned a Doctor of Pharmacy from the University of North Carolina at Chapel Hill. He completed a post-doctoral fellowship in cardiovascular pharmacotherapy with the UNC School of Pharmacy and Division of Cardiology.
learn more
MyFXResearch post: Clinical Trial RECONNECT: ZYN002 Gel
Learn more about the RECONNECT trial, now recruiting individuals with full mutation Fragile X syndrome ages 3 to 17 years old.
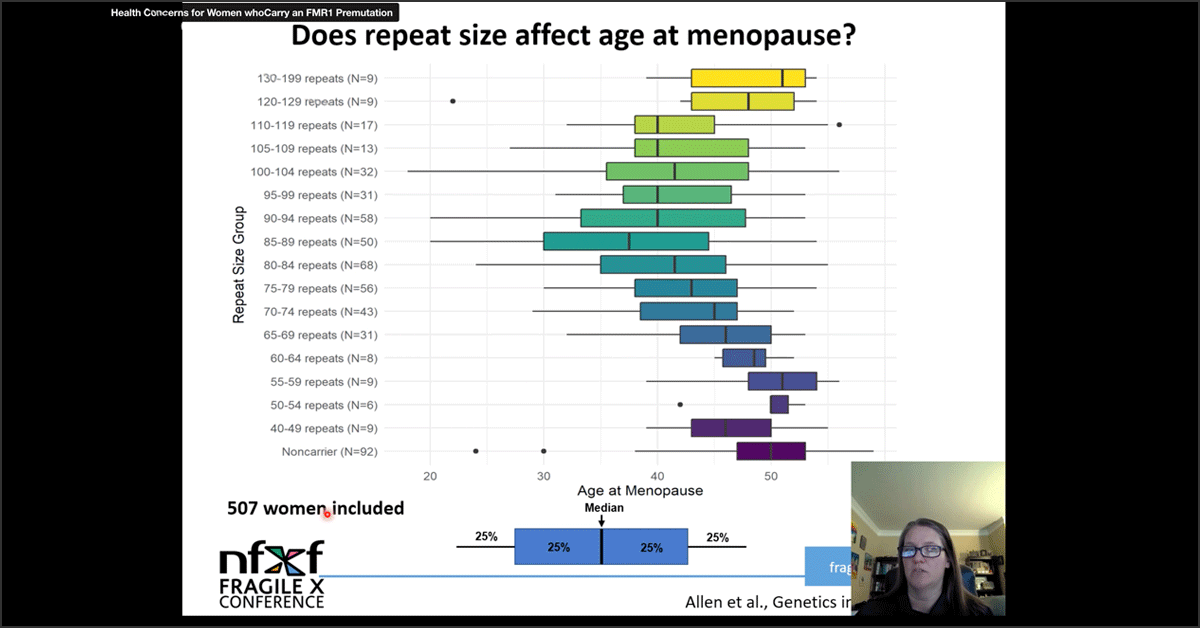
Health Concerns for Women Who Carry an FMR1 Premutation — Presentation
This presentation explores health conditions for women who carry an FMR1 premutation for Fragile X syndrome. Presented by Emily Allen, PhD.
EXPERIENCE Clinical Trials in Fragile X Syndrome
Shionogi's clinical trials for males aged 9–45 living with FXS with a molecular genetic confirmation FMR1 >200 CGG repetitions testing a new drug zatolmilast.