Join Other Rare Disease Advocates in Your District — August 8–19, 2022
It’s that time of year! Join other rare disease advocates for in-district meetings with your Congress members and staff. You still have time to register↗.
Many of you have participated the past few years in in-district meetings with your members of Congress and their staff. These were all scheduled with our partner the Rare Disease Legislative Advocates (RDLA).
This year, we are planning both in-person and virtual meetings. All U.S. House of Representatives meetings will be in-person only. All Senate meetings will be virtual only. Advocates can choose to do both the in-person meeting with their representative and the virtual meetings with their senators.
RDLA organizes all the meetings and helps you prepare by providing legislative resource materials and hosting pre-meeting training webinars. It is all very streamlined and easy on you!
It is always powerful to advocate for Fragile X, both during our own Advocacy Day and in-district with other rare disease communities.
Registration closes July 8, 2022.
We will be providing Fragile X-specific asks via handout as the meetings get closer.
Happy advocating!
about

Hilary Rosselot
Hilary joined the NFXF team in 2019. Prior to joining the NFXF team, she worked at the Cincinnati Fragile X Research and Treatment Center for over five years. She has experience as a clinical research coordinator across many types of clinical trials and served as the clinical research manager for the Cincinnati program. She earned a bachelor’s degree in psychology, a master’s, and is a SOCRA certified clinical research professional (CCRP). She enjoys time with family and friends, a great book, a strong cup of coffee and, of course, a good laugh!
learn more
Fragile X Advocacy
Learn more about NFXF’s Advocacy program, prior asks, and the impact on Fragile X.
Fragile X Advocacy Newsletter – November 2023
Read and share the latest edition of the Fragile X Advocacy Newsletter with your Members of Congress offices!
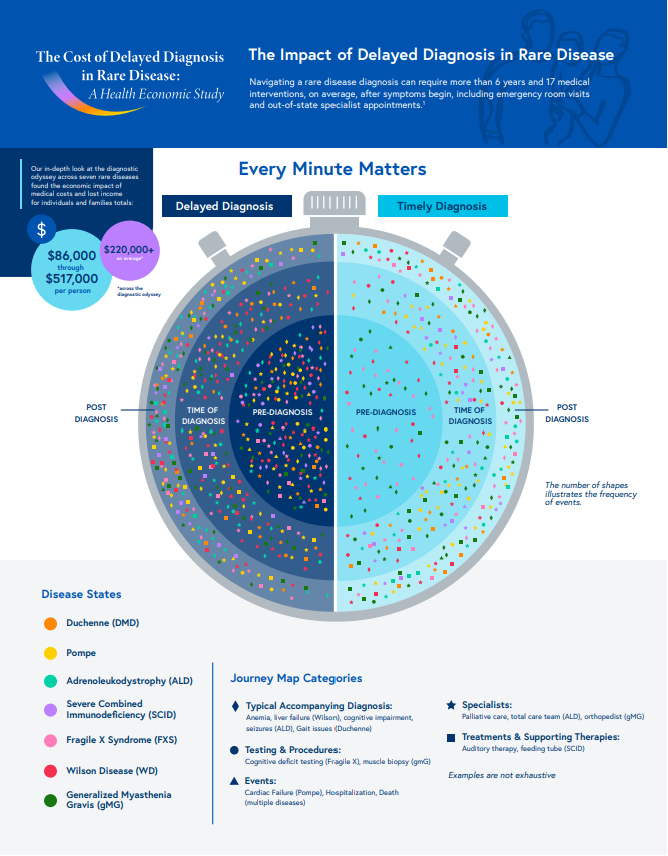
Cost of Delayed Diagnosis in Rare Disease: A Health Economic Study
The National Fragile X Foundation is proud to be part of the Everylife Foundation’s newest report, The Cost of Delayed Diagnosis in Rare Disease: A Health Economic Study.