Western Massachusetts Fragile X 8th Annual Hadley Corn Hole Championship
The 8th Annual Hadley Corn Hole Championship, organized by the Western Massachusetts Fragile X chapter on March 3, 2024, marked a day of camaraderie, spirited competition, and impactful fundraising.
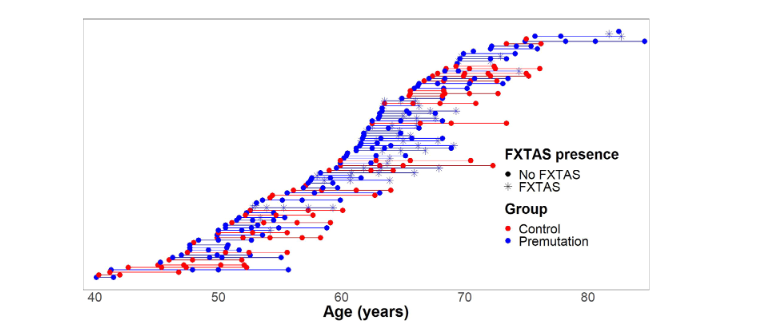
FMR1 Carriers Report Executive Function Changes Prior to Fragile X-Associated Tremor/Ataxia Syndrome: A Longitudinal Study
Authors: David Hessl, PhD, Karina Mandujano Rojas, BS, Emilio Ferrer, PhD, Glenda Espinal, BS, Jessica Famula, MS, Andrea Schneider, PhD, Randi Hagerman, MD, Flora Tassone, PhD, and Susan M. Rivera, PhD Summary: People with [...]
Study: Mechanisms and biomarkers of disease progression in Fragile X-associated tremor/ataxia syndrome (FXTAS)
The University of Kansas BRAIN Lab is conducting a research study to learn about behavioral and brain differences associated with the Fragile X premutation. Males and females ages 50-80 living with the Fragile X premutation, with or without FXTAS, may be eligible to participate. The study includes remote & in-person visits at the University of Kansas.
Peer-Reviewed Medical Research Program’s FY 24 Funding Opportunities for Researchers — Webinar
Dr. Kathryn Argue share tips for applying for PRMRP funding with Fragile X professionals.
The Greater Chicago Fragile X Families Raise Awareness at the Brookfield Zoo Tree Trim
The Greater Chicago Fragile X chapter families gathered on a sunny day in November to support the Brookfield Zoo and its annual Tree Trim event, and to raise awareness for Fragile X families everywhere.
How the NFXF has Informed the Community Through the Years
The NFXF has continually adapted to the times when it comes to the best ways to communicate with the Fragile X community.
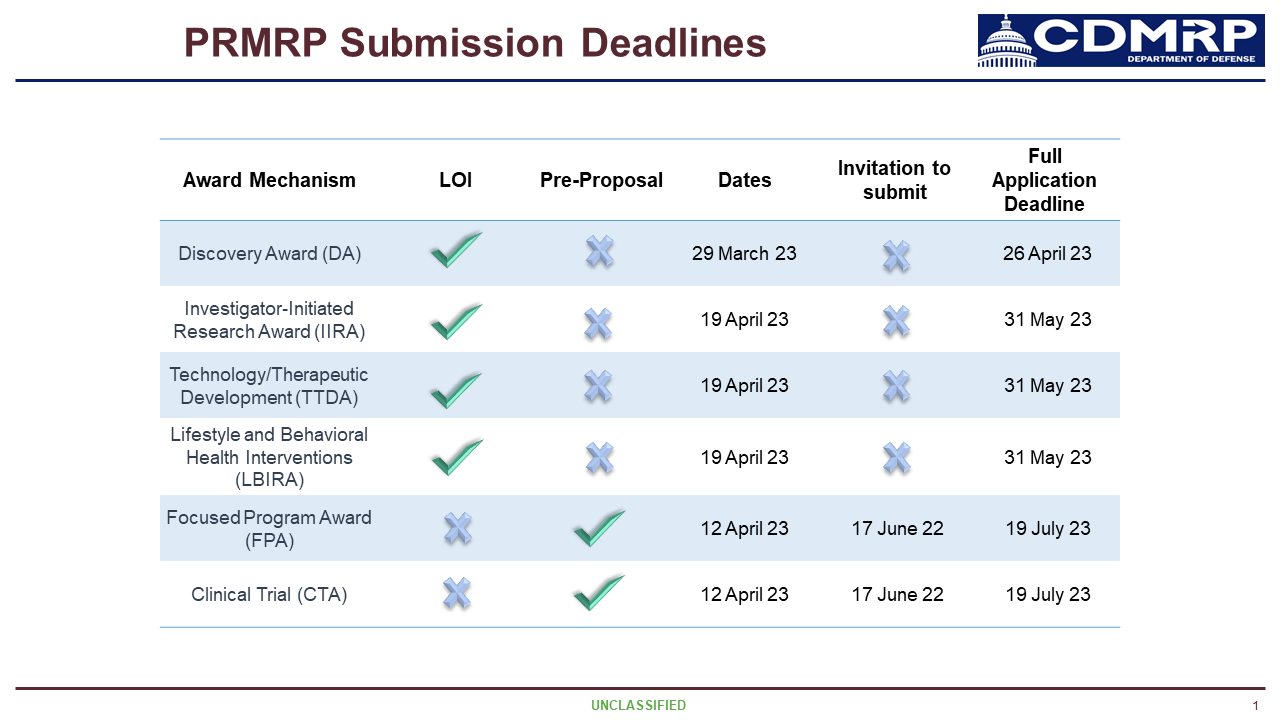
Research Funding Opportunity from CDMRP/PRMRP for Fiscal Year 24
Announcing FY 2024 federal research funding opportunities across six award categories available for all Fragile X-associated conditions and disorders.
Exploring Potential Barriers to the Fragile X Syndrome Cascade Screening Process
Are you an adult with a family member who has been diagnosed with Fragile X syndrome (FXS) or with the FXS premutation? Help us learn about your experiences with the FXS screening process. Researchers [...]
The 1990’s and the NFXF Becoming a True National Organization
Thankfully, we’ve come a long way since the day when FXS was jokingly referred to as “Fragile WHATSyndrome?!” In the 1990’s, many changes were taking place as a result of the increased scientific study of Fragile X and how the growing body of knowledge was impacting the work of the Foundation.
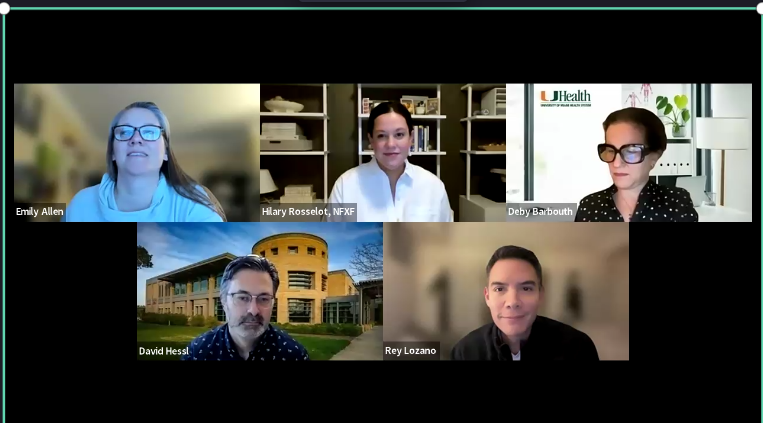
I Have the Fragile X Premutation…Now What?— Webinar
Drs. Deborah Barbouth, Emily Allen, Reymundo Lozano, and David Hessl joined us for a one-hour Q & A discussing the Fragile X Premutation.
Sensory Symptoms and Signs of Hyperarousal in Individuals with Fragile X Syndrome
FORWARD // Researchers conducted the first comprehensive analysis of characteristics of sensory symptoms in children with FXS and their impact on families.
Grief and Bereavement Experiences of Children with Intellectual Disabilities
Researchers at the University of Maryland are looking to understand how children with intellectual disabilities grieve the loss of a loved one. Researchers are currently gathering information on how children with intellectual disabilities experience [...]