Reproductive Health and Your Options
There is no one way to grow a family. Whether you know you carry a gene that can be passed on to your future children and want to explore your options or you simply don’t know where to start, we’re here to help.
We will walk through general tips for family planning as well as the details of carrier screening, the reproductive options that exist, and their associated costs. Each state, clinic, and provider are different, so we strongly encourage you to speak with your doctors, clinics, or other agencies to learn the details of all the options available to you.
We’ve gathered definitions of some common terms you’ll run into here and in your interactions with doctors and other professionals:
Autosomal recessive
Both parents have to have a DNA change in the same gene for a child to be at risk.
Biopsy
An embryo biopsy is removing one cell or a small representative group of cells from a developing embryo. These cells are used for preimplantation genetic testing.
Carrier screening and fertility care
Various healthcare professionals may be involved in carrier screening and fertility care and you may interact with a primary care physician, OB/GYN, or a fertility specialist (e.g., reproductive endocrinologist).
Carrier testing/screening
A type of genetic test that can identify whether you have an increased risk of having a child with a genetic disorder. The testing screens for changes in your DNA that can cause health risks for a child. Some of these are X-linked (e.g., Fragile X), which are passed down from the mother. Some of these are autosomal recessive, which requires both the mother and the father to carry a change in the same gene for a child to be affected.
Embryologist
A professional who is responsible for handling and conducting various tests on reproductive tissue, such as sperm, eggs, and embryos.
Full mutation Fragile X
Above 200 CGG repeats on the FMR1 gene. Individuals with full mutation Fragile X have Fragile X syndrome (FXS).
Fragile X premutation
Between 54 and 200 CGG repeats on the FMR1 gene. Individuals with the Fragile X premutation are sometimes called Fragile X carriers. These individuals are at risk for Fragile X-associated conditions.
In vitro fertilization (IVF)
An assisted reproductive technology in which the ovaries are stimulated with hormones, eggs are retrieved from the ovaries, fertilized outside the body in a laboratory with sperm, and embryos are transferred back into the body with the intention of pregnancy.
Monogenic
Conditions are caused by mutations (DNA change) in a single gene.
Preimplantation genetic testing (PGT)
A screening test that utilizes a few cells from each embryo to identify genetic abnormalities.
Preimplantation genetic testing for aneuploidies (PGT-A)
Screening embryos for chromosome abnormalities (e.g., the number of chromosomes in the embryo).
Preimplantation genetic testing for monogenic/single gene defects (PGT-M)
Screening embryos to identify whether they carry a specific genetic mutation.
Ultrasound
An imaging method that uses sound waves to produce images of the inside of the body. Ultrasounds are used to view things like the uterus and follicles during IVF, or fetuses during pregnancy.
X-linked
A child has a risk of the disease if their mother is a carrier.
From Our Blog
Reproductive Health — Webinar
Drs. Heather Hipp and Victoria Wilkins joined us for an informative webinar on the topic of Reproductive health. This is a broad topic, covering the physical and emotional components of areas like puberty, sexuality, reproductive options, and family planning.
One Fragile X Experience: Carrier & Mom Answers FAQs — Video
Ilana Garber, a Fragile X carrier and mother of a child with Fragile X syndrome, shares her experience with Fragile X and offers her perspective to people interested in testing for themselves or their child.
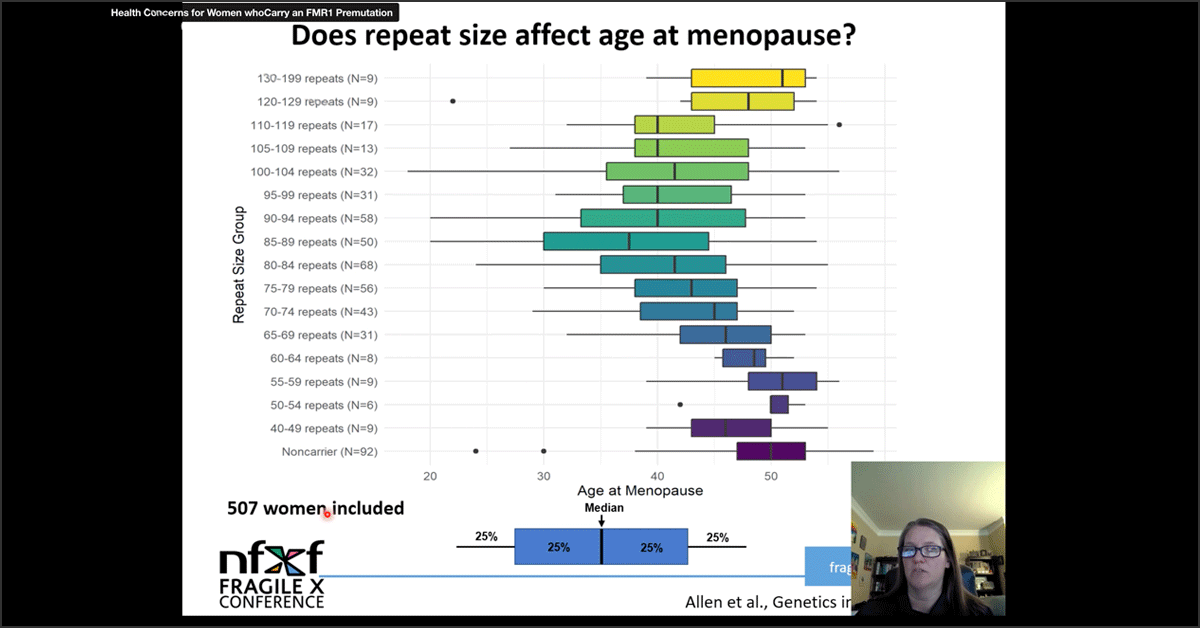
Health Concerns for Women Who Carry an FMR1 Premutation — Presentation
This presentation explores health conditions for women who carry an FMR1 premutation for Fragile X syndrome. Presented by Emily Allen, PhD.
Reproductive and Gynecologic Care for Women with the Fragile X Premutation — Presentation
Care for women with FXPOI who struggle with infertility and the health effects of early onset ovarian insufficiency, including hot flushes, night sweats, and risks of osteoporosis. With Heather Hipp, Jennifer Barber, and Keiko Mathewson.
From Our Blog
Reproductive Health — Webinar
Drs. Heather Hipp and Victoria Wilkins joined us for an informative webinar on the topic of Reproductive health. This is a broad topic, covering the physical and emotional components of areas like puberty, sexuality, reproductive options, and family planning.
One Fragile X Experience: Carrier & Mom Answers FAQs — Video
Ilana Garber, a Fragile X carrier and mother of a child with Fragile X syndrome, shares her experience with Fragile X and offers her perspective to people interested in testing for themselves or their child.
Health Concerns for Women Who Carry an FMR1 Premutation — Presentation
This presentation explores health conditions for women who carry an FMR1 premutation for Fragile X syndrome. Presented by Emily Allen, PhD.
Reproductive and Gynecologic Care for Women with the Fragile X Premutation — Presentation
Care for women with FXPOI who struggle with infertility and the health effects of early onset ovarian insufficiency, including hot flushes, night sweats, and risks of osteoporosis. With Heather Hipp, Jennifer Barber, and Keiko Mathewson.
Tips for Family Planning
There is a lot of information to sift through as you choose to grow your family. It can be an emotional process that comes with highs and lows. If we could give you overarching tips for success, these would be it:
1. Understand Your Fragile X Status
Individuals have the option to participate in carrier screening. Knowing your Fragile X status as well as your carrier status for other genetic conditions (e.g., sickle cell disease, cystic fibrosis) can help inform your healthcare, and, potentially, your family planning goals.
2. Partner With a Fantastic Doctor or Clinic
You will have to be your advocate throughout this process, and you should partner with a clinic or doctor (or both) who is knowledgeable about Fragile X, listens to you, and makes you feel comfortable. Having a clinic that supports your goals makes a huge difference!
3. Identify a Support Network
Having a supportive network makes a huge difference. You may want to see a therapist, share your journey with trusted friends or family, or seek out others who are on similar paths. Knowing you are not alone and having people to lean on will come in handy!
4. Discuss Your Options
Discuss your options with your doctor. Understanding your options and knowing you have a choice is important. If you have questions, ask! There are no stupid or silly questions.
5. Understand Any Health Implications
There may be health implications to being a carrier for a genetic condition and for undergoing various fertility treatments. Discuss these options with trusted healthcare professionals to know your risks and assess them for yourself.
6. Do What’s Right for Your Family
It’s your life! What works for you may not work for someone else. There is no perfect answer or one-size-fits-all approach. Making informed decisions that honor your personal beliefs is key.
7. Seek Financial Support
Adoption, IVF, and genetic testing can be expensive. Talk with your insurance provider and employer about coverage and potential benefits. More states have started covering reproductive treatment as part of insurance coverage.
RESOLVE: The National Infertility Association is a fantastic resource to learn about state-by-state coverage and how to advocate for improved employer benefits. Some clinics offer grant programs for IVF. Explore your options!
Carrier Testing & Screening
Carrier testing and screening is a type of genetic test that can tell you whether you carry a DNA change in genes that can cause certain genetic disorders that could affect a child. Carrier screening can be done before or during pregnancy, though preconception counseling and testing are recommended to help families understand their options. Both the female and male partners are eligible for carrier screening.
Some of the diseases on the panels are X-linked and some are autosomal recessive. this means a child has a risk of the disease if their mother is a carrier. Some of the diseases are autosomal recessive, which means both parents have to have a DNA change in the same gene for a child to be at risk.
If an individual is found to be a carrier for a specific condition that is autosomal recessive, subsequent testing of the individual’s reproductive partner can then be performed to receive additional information about possible reproductive outcomes, and options for additional testing.
While carrier screening should always be offered, it is never required. Carrier screening may or may not be covered by your insurance.
A sample of blood, saliva, or tissue is needed to perform carrier testing and screening. There are many options for carrier testing and screening, including targeted carrier screening or expanded carrier screening.
Targeted carrier screening is carrier screening based on your family’s history or ethnicity. For example, if you have a family history of Fragile X, you may just want Fragile X carrier testing or, if you are African American, you may want to receive carrier screening for sickle cell disease as it predominantly affects people of African descent.
Expanded carrier screening is a larger panel, meaning you will be tested for being a carrier for many genetic conditions and disorders at once. Your healthcare provider likely works with a specific carrier screening company, and each company creates its own expanded carrier screening list.
Here is a short video overview of carrier testing and screening:
JScreen’s ReproGEN Educational Video
Video from MyJScreen, a non-profit, community-based public health initiative dedicated to preventing Jewish genetic diseases and promoting healthy families.
Even if you know you have the Fragile X premutation (commonly referred to as being a Fragile X carrier) or full mutation, you may still want to consider expanded carrier testing to understand your risk for other genetic conditions.
Your healthcare provider may receive your results first and they will review the results with you or suggest you speak with a genetic counselor. Not all genetic conditions and disorders are inherited or passed on like Fragile X; the genetics and inheritance patterns of Fragile X are complex. It is very important to understand the details of your results with a healthcare provider or genetic counseling professional.
EXTERNAL RESOURCES
Screening for Autosomal Recessive and X-Linked Conditions During Pregnancy and Preconception: A Practice Resource of the American College of Medical Genetics and Genomics (ACMG)
American College of Medical Genetics and Genomics
ACMG Recommendations for Preconception and Prenatal Carrier Screening
American College of Medical Genetics and Genomics ObG Project
Contraception
Note: We understand contraception is not for everyone. If you are not interested or do not believe in the use of contraception, feel free to skip this section.
There are many options available for birth control if you are not ready or not planning to be pregnant. Sorting through all of the options available for birth control may be a bit overwhelming, but it is important to make an informed decision on what option might work best for you. Factors to consider when weighing your options include:
- How effective is this method?
- Does this method protect against sexually transmitted infections (STIs)?
- How often do you need to remember to utilize this method?
- Is it practical for your lifestyle?
- Is this method reversible?
- What are the potential side effects?
- Do you have any medical diagnoses that could make a method higher risk?
- Is this method affordable?
All methods of contraception have their pros and cons. Prioritizing those things that are most important for your family planning goals and lifestyle, in addition to speaking to your medical provider, will help you decide which method will work best for you. Even if your initial method is not a perfect fit, do not be discouraged! There are many options from which to choose.
Combined Hormonal Birth Control Methods
Hormonal birth control works to prevent pregnancy by various methods, though most pause the body’s monthly ovulation process (the release of an egg from the ovary). Hormonal methods include a birth control pill (“the pill”), a patch that is applied to the skin, and a vaginal ring.
BIRTH CONTROL PILL
When taken as directed, the birth control pill is very effective at preventing pregnancy. With this method, it is best to take the pill at the same time every day so that it is a part of your routine. If you happen to miss a pill, make sure to read the package instructions to plan your next course of action. This method is 93% effective with typical use.
SKIN PATCH
The skin patch is a 1.75-inch adhesive patch that is applied to the skin (upper arm, shoulder, upper back, hip) once a week. The patch is applied weekly and on the fourth week, the patch is left off to allow for a period. The skin patch contains similar hormones to those found in birth control pills. The patch can still be worn when exercising, bathing, or swimming. This method is 93% effective with typical use.
VAGINAL RING
The vaginal ring is a small, flexible ring that is inserted into the upper portion of the vagina. The ring stays in place for 21 days, after which it is removed for seven days to allow for a period. The ring contains hormones similar to both the birth control pill and the skin patch. It is okay to have intercourse with the ring in place. This method is 93% effective with typical use.
DEPO-PROVERA
The only injectable version of birth control, also known as “the shot,” is Depo-Provera. This is a long-lasting progesterone hormone that is given as an injection every three months to prevent pregnancy.
The shot is injected into the muscle of the buttock or upper arm, similar to most seasonal vaccines. It can take up to 6-12 months for menses to return following stopping this medication; it is not recommended for individuals who are thinking about pregnancy within the next year. It is 96% effective with typical use.
BARRIER METHODS
Barrier contraceptives prevent sperm from entering the uterus. These methods included the external condom, internal condom, diaphragm, and cervical cap. Correct use of these devices is important to ensure effectiveness.
As opposed to other methods, barrier contraceptives can decrease the transmission of sexually transmitted infections (STIs). They can be also used in addition to the other methods discussed here to lower the chance of contraception failure. These methods can vary from 71% to 87% effective with typical use.
INTRAUTERINE DEVICE
The intrauterine device is a small T-shaped device that is inserted into the uterus by your healthcare provider. The insertion does require a procedure performed at the doctor’s office; however, this method can last for years (approved duration varies depending on type, but most are 5-7 years) and does not require the user to remember to take or place any medication.
There are two main types of IUDs — those that contain a hormone (progesterone), and one that does not contain hormones (copper IUD). Their effect on menstrual cycles also varies depending on type. This method is 99.2–99.9% effective.
IMPLANT
The Nexplanon device is a small implant, about the size of a matchstick, that is inserted into the upper arm by your healthcare provider. The implant is not apparent following placement. It is another progesterone-only method that can prevent pregnancy for up to five years. Its effect on menstrual cycles varies from person to person, though it can be associated with irregular bleeding. This method is 99.9% effective.
EMERGENCY CONTRACEPTION
Emergency contraception (EC) can stop a pregnancy before it starts — in a situation where no birth control was used, or if there is a concern that the selected method of birth control may have failed or not been used appropriately. Time is of the essence when considering these methods, as their effectiveness declines with time.
There are two oral medications available for emergency contraception. One option is levonorgestrel-based pills (often called “Plan B”). These pills do not require a prescription and can be purchased over the counter. This medication can be taken up to five days after unprotected intercourse, though the effectiveness is best within the first three days. These medications are not as effective for women who weigh more than 165 pounds.
Alternatively, the other oral medication available for emergency contraception is ulipristal acetate (Ella). This medication requires a prescription from your provider and has stable efficacy up to five days after unprotected intercourse. There is also no decrease in effectiveness in weights above 165 pounds. These oral EC methods have an efficacy rate of 85%–99%.
Lastly, the non-hormonal copper IUD (ParaGard) can be inserted into the uterus up to five days after unprotected intercourse — but this method does require a doctor’s appointment for placement of the device.
EXTERNAL RESOURCES
Bedsider
A site that provides information on birth control for people ages 18 to 29 years. Run by the nonprofit Power to Decide.
Patient Education: Birth Control; Which Method is Right For Me? (Beyond the Basics)
UpToDate
Reproductive Health: Contraception
CDC
How Well Does Birth Control Work?
The Regents of the University of California
Growing Your Family with Fragile X Syndrome or Fragile X Premutation
Couples may find out that the female or the male partner has an expansion on the FMR1 gene, including the Fragile X premutation, commonly referred to as a “Fragile X carrier” or the full mutation referred to as “Fragile X syndrome.” Naturally, many questions arise.
Commonly asked questions include, What are my options for the future? How can I increase my chance of having a child without Fragile X? What are the options to test a pregnancy in the future?
Growing your family is not always a straightforward process, and depending on your personal, cultural, and religious beliefs, some options may not be the right fit for you. There are several ways to build a family when one of the partners has the Fragile X premutation or full mutation. Some of these options include:
1. Conceiving naturally and not doing any testing.
2. Conceiving naturally and testing the pregnancy via prenatal diagnostic tests.
3. Undergoing in vitro fertilization (IVF) and using:
- both partner’s genetic material (egg from female and sperm from male), testing any embryos in the laboratory, and implanting only unaffected embryos.
- a donor egg (if the female partner carriers the premutation or has Fragile X syndrome).
- donor sperm (if the male partner carriers the premutation or has Fragile X syndrome)
- a donor embryo, which would have no genetic material from either partner.
4. Choosing to adopt.
5. Choosing not to have children.
Let’s walk through each of these options together.
1. Conceiving Naturally and Not Doing Any Testing
You and your partner may choose not to have any type of genetic screening and testing for yourselves or your future child or children. This is your choice, you do not have to do any testing.
All carrier and genetic screening and testing are optional. If you change your mind, any screening or testing can be performed later in life at the recommendation of a healthcare provider. If you choose not to test yourself and your child’s physician is concerned about a potential genetic disease in your child, they can order necessary testing after the child’s birth.
2. Conceiving Naturally and Testing During the Pregnancy
There are two different methods to evaluate the genetic status of the fetus during pregnancy:
- Amniocentesis
- Chorionic villus sampling (CVS)
Your healthcare provider will discuss and review the procedures, and possible risks, and answer any questions you and your partner may have.
Both procedures obtain tissue or amniotic fluid from the pregnancy to obtain DNA. The DNA is then analyzed to determine the genetic status of the pregnancy. It can test for the number of chromosomes and specific genetic disorders. These tests are commonly offered to women for reasons like advanced maternal age or a positive carrier screening test. You can speak with your healthcare provider about these options if you know your Fragile X status and would like to know the Fragile X status of the fetus.
AMNIOCENTESIS
Amniocentesis, sometimes called an “amnio,” uses a small sample (two tablespoons) of the amniotic fluid that surrounds the fetus. This fluid has fetal cells that can be grown and analyzed for various genetic conditions.
The procedure is usually performed between 15 and 20 weeks of gestation, which is determined by ultrasound or by the first day of the last menstrual period. Using an ultrasound, the healthcare provider inserts a very thin needle through the abdomen to withdraw the fluid from the uterus. The fluid is then taken to the laboratory to begin the testing process. For many women, the procedure feels like a blood draw, though some feel mild cramping in their uterus.
After the procedure, the woman is given care instructions regarding rest, travel, etc. The risks of the procedure include miscarriage, bleeding, cramping, and amniotic fluid leakage. The risk for miscarriage is approximately 0.1% to 0.3% when done by a skilled healthcare provider using ultrasound during your second trimester.
Your healthcare provider or a genetic counselor will help you understand your amniocentesis results. It may take a few weeks to receive your results.
CHORIONIC VILLUS SAMPLING (CVS)
Chorionic villus sampling requires obtaining a small sample from the developing placenta.
The placenta is usually the same genetically as the fetus, but there can be exceptions. Chorionic villi are small fingerlike projections on the edge of the placenta. The cells can be studied for chromosome abnormalities such as Down syndrome and FXS if the woman is known to have the Fragile X premutation or full mutation.
CVS is performed earlier in pregnancy than amniocentesis, usually between 10 and 13 weeks of gestation. It is also performed using ultrasound equipment.
There are two methods of withdrawing the sample, depending on the position of the placenta.
- If the placenta is behind the fetus, the healthcare provider inserts a thin tube through the vagina/cervix.
- If the placenta is in front of the fetus, a small needle is inserted through the abdomen, similar to amniocentesis.
Using gentle suction, the doctor removes a very small amount of chorionic villi for laboratory analysis. Some women feel cramping or pressure, while others do not find it uncomfortable. Like amniocentesis, there is a small risk of miscarriage (less than 1%). If performed earlier than 10 weeks gestation, there is an associated risk of limb defects.
3. Undergoing In Vitro Fertilization (IVF)
In vitro fertilization (IVF) is an assisted reproductive technology in which the ovaries are stimulated with hormones to produce multiple eggs, the eggs are retrieved and fertilized with sperm outside the body in a laboratory. Once the eggs are fertilized, they may or may not become embryos.
If they become embryos, you can choose to have them undergo genetic screening before any embryo transfers and pregnancy. The subsequent embryo transfer can happen the following month or the embryos can remain frozen for multiple years (e.g., for family building). Following the embryo transfer, you will wait about two weeks to take a pregnancy test.
Individuals considering IVF are encouraged to check with their insurance provider about coverage before initiating any services. Some employers may also offer employee assistance programs that support fertility services and adoption. Many IVF clinics also have service and financial navigators that you may be able to speak with.
RESOLVE: The National Infertility Association is a fantastic resource to learn about state-by-state coverage and how to advocate for improved employer benefits.
EXTERNAL RESOURCES
Preimplantation Genetic Testing
American College of Obstetricians and Gynecologists
CHOOSING A CENTER
You can explore fertility clinics nationwide and their respective success rates through the Society for Assisted Reproductive Technology.
You might consider asking the clinic the following questions:
- Have you heard of Fragile X?
- How much experience do you have with Fragile X testing?
- Do you have a genetic counselor on staff?
- What lab does your clinic use for embryo genetic testing?
- Do you have a financial counselor or consultant to help understand the cost/expense?
- How many “cycles” are typical for most patients?
- What are your success rates and failure statistics? Do you have this information specific to those individuals with Fragile X?
- What testing should I have done to have a better idea of our potential success chances?
- Do you have additional or alternative therapies that you recommend (e.g., acupuncture, dietary, yoga, supplements)?
- If applicable, how will my FXPOI impact this process?
- What are realistic expectations based on what you know about my or our health history?
STARTING THE IVF PROCESS
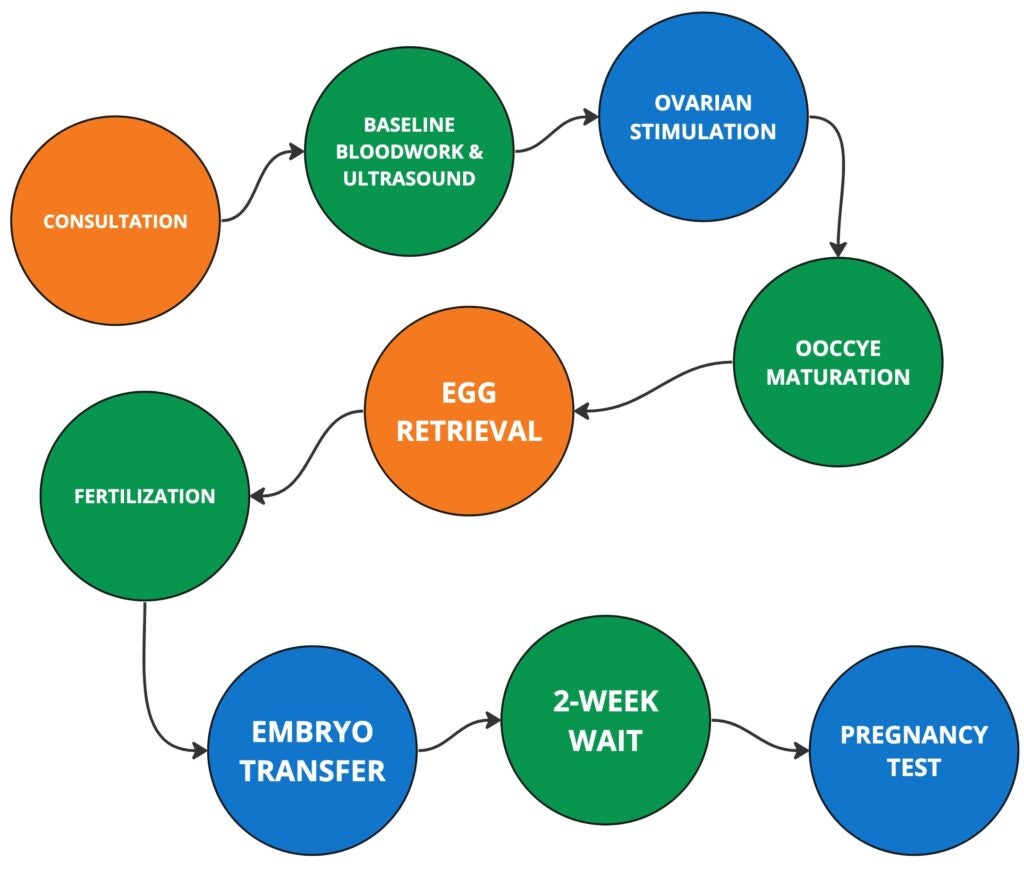
CONSULTATION
Before any egg retrievals or embryo transfers, you must have a fertility evaluation. For females, an ovarian reserve quantity of eggs is checked, and for males, sperm count and quality are assessed.
You will consult with a fertility specialist, likely a reproductive endocrinologist, on your goals. This is a great time to explain your Fragile X status and if it has an impact on your family planning.
Genetic pre-clinical workup (test development process): For those electing to pursue PGT-M to test for monogenic conditions (i.e., Fragile X syndrome), each partner’s DNA needs to be understood. To develop the custom test information (often referred to as a “probe”), DNA samples (blood or saliva) are collected from each partner to create a custom “fingerprint” associated with the genetic expansion. In some situations, additional DNA samples of family members may be requested.
Questions you may consider discussing during the consultation:
- Who in our family will be requested to provide DNA samples?
- What if the family member is unable to provide a DNA sample, will the accuracy be impacted?
- How long will the test development process take once the DNA samples are received?
- Is there an additional cost or fee for the test development process?
Many patients who are biopsying their embryos for PGT-M for a specific disease also choose to screen the same sample for the number of chromosomes, which is called “aneuploidy screening” (PGT-A).
EXTERNAL RESOURCES
Spectrum PGT-M Patient Brochure
Natera
Preimplantation Genetic Testing for Monogenic/Single Gene Defects (PGT-M)
ORM Genomics
OVARIAN STIMULATION
Once the consultation and evaluations are complete, you will begin hormone medications to stimulate your ovaries before the egg retrieval.
The medications typically start on Day 1 of your cycle (the first day of your period). The medications are all injectable (e.g., shots) hormones that patients give themselves at home. Routine monitoring will occur leading up to the retrieval and includes blood draws to test your hormone levels and transvaginal ultrasounds.
Typically, you only ovulate one egg per cycle. Ovarian stimulation attempts to grow as many eggs as possible in a safe way, which may be uncomfortable. Tell your fertility specialist if you become uncomfortable or are in pain at any time in the process.
Your fertility specialist will advise you on any medications you will need and how to take them to prepare for the egg retrieval. You will have frequent appointments for monitoring while you take medication.
Once your fertility specialist determines your follicles — or the structures where an egg grows — are large enough, you will “trigger” them. This trigger is the last piece to help the eggs fully mature before retrieval. This trigger is usually a shot, and the timing of this shot is very important. Be sure you know the exact time you are supposed to take the shot before the egg retrieval.
After your trigger shot, you will be scheduled for your retrieval.
EGG RETRIEVAL
Your retrieval is two days after your trigger shot. Egg retrievals are typically done under light sedation or anesthesia.
TIP Speak with your fertility specialist about any concerns with any anesthesia. You could also consider sharing this article: General Anesthetic Use in Fragile X Spectrum Disorders from the NIH.
Once you are comfortable, your fertility specialist will use a transvaginal ultrasound to guide a small needle through the vagina into your ovaries. Using the needle, your fertility specialist will take the fluid and egg from each of your follicles. The fluid is then looked at by the embryologist, who can identify the egg or eggs under a microscope and will perform fertilization of the eggs in the lab.
A sperm sample will be used for fertilization and is typically collected on the day of the egg retrieval. Frozen sperm samples can be used, though fresh are preferred.
Once the egg retrieval is complete, you will be taken back to a recovery area. You may experience some cramping or spotting after the procedure, drowsiness, or fatigue, though you will likely feel back to normal in a day or two. Ask your fertility specialist about what to expect!
Some individuals need to go through the ovarian stimulation and egg retrieval process more than once. A few factors that may influence this process include your family-building goals, response to the stimulation, or how the embryos grow in the lab. Understanding your possible outcomes is a great discussion to have with your doctor before beginning IVF.
FERTILIZATION
An embryologist will combine any mature eggs with sperm for fertilization. Approximately 70% of eggs will fertilize. Embryos are monitored for the next three to five days. The embryologist will then prepare an embryo for a fresh embryo transfer, freeze the embryos, or prepare the embryos for genetic screening (preimplantation genetic testing).
Sometimes additional processes may be used to support fertilization or implantation. Examples of these processes are intracytoplasmic sperm injection, or ICSI, in which the embryologist uses a single sperm and injects it into each egg; or assisted hatching, in which the embryologist helps the embryo hatch from its shell, which may help the embryo implant. These are not always necessary, recommended, or covered by insurance.
EXTERNAL RESOURCES
IVF (In Vitro Fertilization)
Cleveland Clinic
PREIMPLANTATION GENETIC TESTING (PGT) — Optional
PGT Testing Procedures: A few cells from each embryo can be sent for preimplantation genetic testing (PGT) for either monogenic disorders (PGT-M) or the number of chromosomes (aneuploidy) (PGT-A).
PGT-M is used to screen for a specific gene or genetic condition like Fragile X. PGT-M tests a single embryo for the condition for which you specifically want to be tested, it does not test for all possible genetic conditions. Having carrier screening ahead of time can help make sure the risk of other potential genetic diseases is known before IVF.
PGT-A does not screen for specific genes or genetic conditions like Fragile X. PGT-A assesses the overall chromosomal makeup of the embryo.
- If an embryo has 46 chromosomes — 23 from the female and 23 from the male — it is considered an euploid embryo.
- If there is an extra chromosome or is missing a chromosome, that embryo is considered an aneuploid embryo.
The chance that each embryo is euploid depends on the woman’s age. Discuss with the healthcare team what is the most appropriate testing option for you. There are challenges associated with conducting both PGT-M and PGT-A.
Should you choose to undergo PGT-M testing for Fragile X, they can select PGT-M with triplet repeat detection, which identifies both the full mutation (FXS) and the premutation of the FMR1 gene. These tests have their limitations, and you must discuss the risks and benefits of them with your healthcare provider.
One of the difficulties when attempting PGT for individuals with the Fragile X premutation is that some individuals have Fragile X-associated ovarian insufficiency (FXPOI), which can range from premature menopause to reduced ovarian functioning. This can make it difficult to stimulate the ovaries to make the eggs needed for PGT.
TIP Women with a full mutation are not at risk for FXPOI and have no known associated infertility.
The overall accuracy of PGT-M is >97%; therefore, diagnostic prenatal testing by CVS or amniocentesis is often offered later in pregnancy.
PGT Results: If you elect to complete PGT-M on your embryos, testing will assist with identifying embryos that have inherited an FMR1 expansion resulting in the full mutation or premutation. Once evaluation and testing have been completed, the fertility specialist or genetic counselor will discuss the results with you. The fertility specialist may offer recommendations and suggestions based on the PGT results, but ultimately the decisions moving forward are up to you and your partner.
EMRBYO TRANSFER
Embryo transfers are either “fresh” or “frozen,” If you choose to do PGT, your embryos will be cryopreserved or frozen until the testing results are in. This can take 2-3 weeks. If you choose not to do PGT, you may be eligible for a fresh transfer, meaning your fertility specialist will schedule an embryo transfer a few days after your egg retrieval.
Both frozen and fresh transfers follow the same process. Most processes involve taking medications to prepare the uterine lining leading up to the transfer.
An embryo transfer does not require anesthesia and typically takes less than 10 minutes. Your fertility specialist will use a speculum and insert a catheter through your cervix into the uterus. The embryo will travel into your uterus through the catheter. You may experience mild cramping or spotting in the days following the transfer. You will typically be scheduled for a pregnancy test around two weeks later.
What about the remaining embryos? It is important to discuss your options for the embryos you may not be able to use with your fertility specialist. Your decision may vary according to personal beliefs and financial considerations and some options are not available in every state due to state law. Keeping an open line of communication with your partner and fertility specialist before and throughout the process is important in helping you to make the best decision for you. Working with a psychologist who specializes in fertility issues may also help. Your options may include:
- Embryo donation: Couples may choose to donate their fertilized embryos to other couples who are unable to produce embryos on their own.
- Donate embryos to scientific research: Couples may donate unused embryos to scientific research.
- Thaw and dispose of embryos: Couples may choose to thaw and dispose of their unused embryos utilizing their clinic’s resources.
- Do nothing, decide later: Embryos may be frozen and stored for a fee at some fertility labs. Other labs may require the patient to relocate their embryos to a commercial storage site until they have determined what option is right for them.
USING DONOR EGGS, SPERM, OR EMBRYOS
Some couples choose to use materials from a donor without the Fragile X premutation or full mutation. Couples can use either donor eggs, sperm, or even a donor embryo. This option may be especially helpful for females with the Fragile X premutation who have FXPOI and may struggle to develop many eggs of their own.
Using donor materials can also eliminate the risk of passing on Fragile X if that is of interest to you. However, if you wish to have a child who is biologically related to both the mother and father, this option does not meet those needs.
The process of utilizing donor eggs also utilizes IVF. The female recipient begins the process with medical tests for any infectious diseases, RH type, thyroid, general health screening, and so on. The egg donor goes through their screening, which includes hormone levels, genetic screening usually including FMR1 (Fragile X) screening, family history assessment, psychological consultation, and more. The recipient couple meets with a donor coordinator to discuss their criteria for a good match, and then, with the coordinator, selects an egg donor whom they feel would meet these criteria.
Following the donor screening and selection, the egg donor is given injectable hormones to stimulate her ovaries to produce multiple eggs. The eggs are retrieved from the donor at the optimal time (called “egg retrieval”), and then the eggs are fertilized in the laboratory by sperm from the recipient’s husband or a donor.
The embryos can either then be frozen for a future transfer to the intended parent’s uterus or their cycles can be coordinated for a fresh transfer. The intended parent usually stays on hormones for two months to support the early stages of the pregnancy.
4. Choosing to Adopt
Couples who wish to grow their family but do not want to use their genetic material, are unable to use their genetic material, or simply want to explore additional options, may choose to adopt. The adoption process can be lengthy, and there is never a guarantee that couples will be able to adopt.
Some couples choose to foster children, which can lead to adoption. Other couples may work with either a public or private agency to identify children who are eligible for adoption.
Speak with your local public agency or one or more private agencies to find the right fit. Public agency services may be free or very low cost and are potentially reimbursable.
Private agencies go through the same processes as public agencies but typically charge for their services. Do your homework. There are, unfortunately, groups and people who take advantage of couples in this space by charging fees up front and then not following through with services. Ask for references or to be connected with other families who they have worked with.
EXTERNAL RESOURCES
National Council for Adoption (NCA)
About Adoption From Foster Care
National Adoption Association
Adoption Explained
Adopt America Network
Adoption Resources
Adopt America Network
4. Choosing Not to Have Children
Some couples may choose not to have children. Growing your family is your choice, and if you decide it is not for you, that is your choice to make, no matter the reasons that inform your choice.
Costs
IVF — with your genetic material or a donor — and adoption can be expensive endeavors. The cost of IVF alone is usually $15,000 to $20,000 or more, with up to $10,000 in additional fees for PGT.
These fees will depend on the number of cycles, number of embryos, other testing (such as chromosome analysis), fresh vs. frozen transfers, medications, etc. Health insurance will sometimes pay for a portion of it — coverage will vary from plan to plan and may depend on if the couple is diagnosed with infertility. Speak with your insurance provider about your coverage.
Some employers offer employee assistance programs that offset the costs of growing a family through fertility services or adoption. Some fertility clinics offer financial counselors and IVF grants as well.
EXTERNAL RESOURCES
Carrier Screening
American College of Obstetricians and Gynecologists
Prenatal Genetic Screening Tests
American College of Obstetricians and Gynecologists
NIPT
NIPT is a non-invasive genetic screening test that can tell you about the genetic health risks of your pregnancy.
In Vitro Fertilization (IVF): What to Expect
Verywell Family
IVF (In Vitro Fertilization)
Cleveland Clinic
PGT-M and PGT-A Genetic Screening Before IVF
Verywell Family
Preimplantation Genetic Testing for Monogenic/Single Gene Defects (PGT-M)
ORM Genomics
National Council For Adoption (NCA)
About Adoption From Foster Care
National Adoption Association
Adoption Explained
Adopt America Network
Adoption Resources
Adopt America Network
EXTERNAL RESOURCES — FOR FAMILIES
Carrier Screening
American College of Obstetricians and Gynecologists
Prenatal Genetic Screening Tests
American College of Obstetricians and Gynecologists
NIPT
NIPT is a non-invasive genetic screening test that can tell you about the genetic health risks of your pregnancy.
Screening for Autosomal Recessive and X-Linked Conditions During Pregnancy and Preconception: A Practice Resource of the American College of Medical Genetics and Genomics (ACMG)
American College of Medical Genetics and Genomics
ACMG Recommendations for Preconception and Prenatal Carrier Screening
American College of Medical Genetics and Genomics ObG Project
Carrier Screening for Genetic Conditions
ASURAGEN
PGT-M With or Without Triplet Repeat Detection for Fragile X Syndrome
World Scientific Publishing
AUTHORS’ NOTE
This information was authored in partnership with Drs. Heather Hipp and Jamie Merkison of Emory University.
Women’s Health and the Fragile X Premutation
You may also be interested in the ebook Women’s Health and the Fragile X Premutation, which was created to empower premutation carriers of all ages and to serve as a guide for women, their families, and medical providers.
This book is organized in a question-and-answer format and is divided into 5 main sections:
- The Fragile X Premutation and its Inheritance in Families
- Fragile X-Associated Primary Ovarian Insufficiency (FXPOI)
- Fragile X-Associated Tremor/Ataxia Syndrome (FXTAS) and Other Premutation Related Health Concerns
- Family Planning
- Health Worksheet to help you discuss the premutation with your doctor
Questions?
If you’re a parent or caregiver and have questions about the information presented here, we’d love to hear from you! You can reach out to Missy Zolecki using the contact info or our contact form below.

Missy Zolecki,
Community Empowerment
treatment@fragilex.org
(800) 688-8765