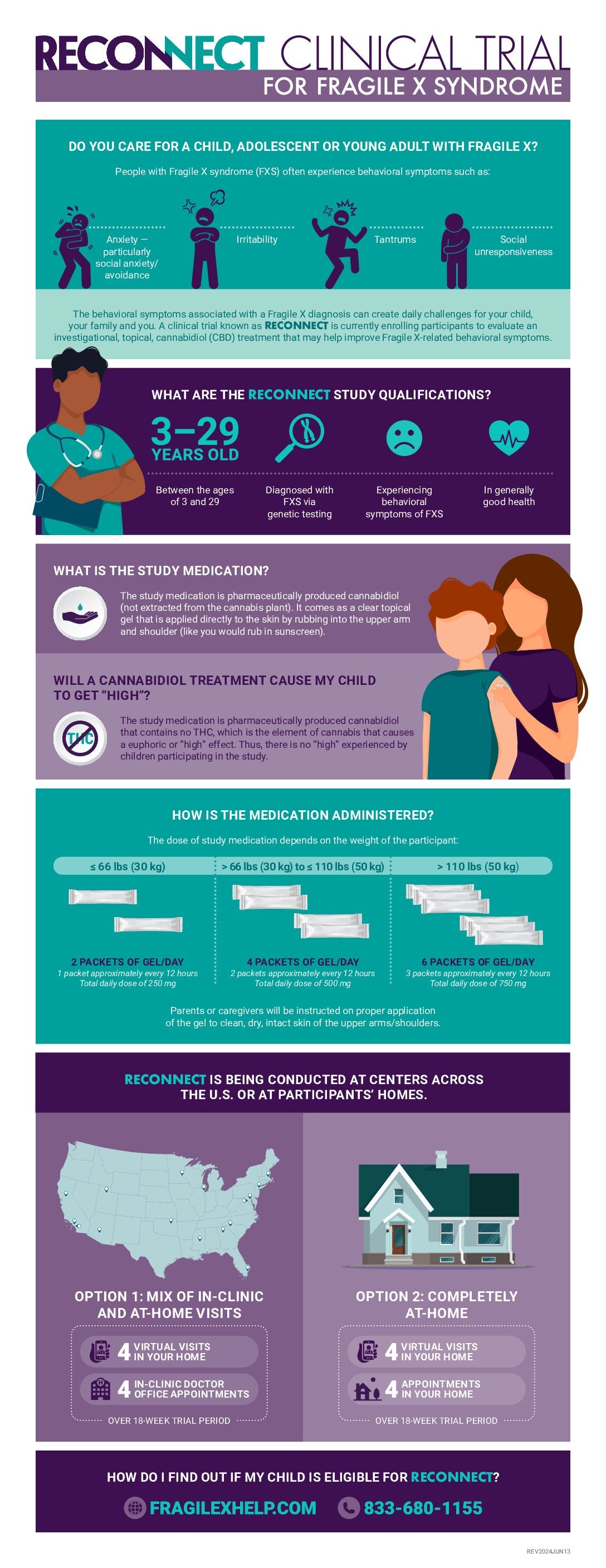
RECONNECT clinical trial is now enrolling males and females from the age of 3 years up to 29 years old!
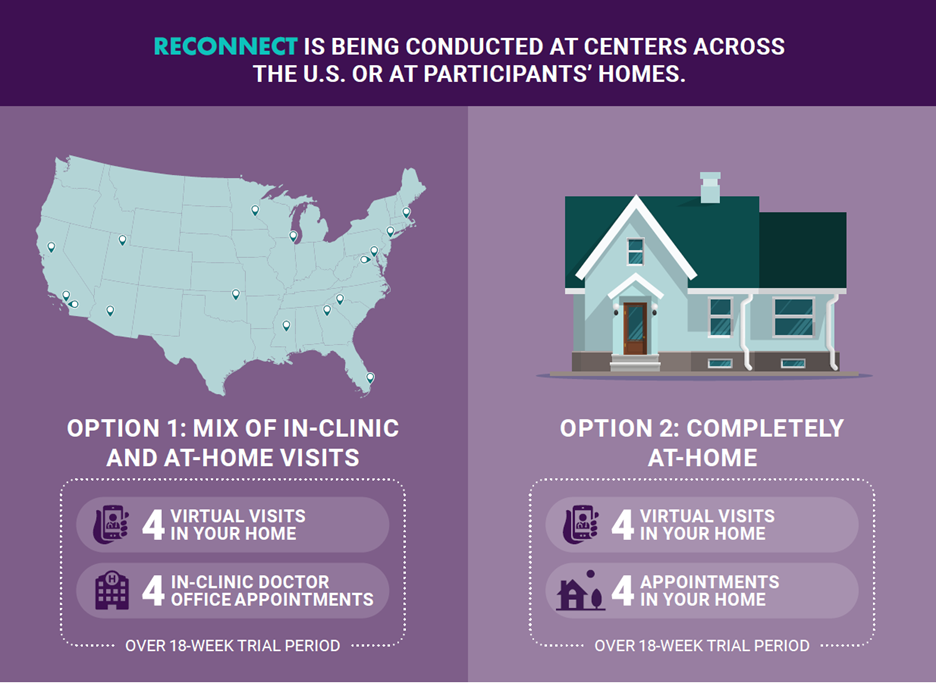
RECONNECT offers 2 options for participation:
Option 1: Mix of In-Clinic and At-Home Visits
Option 2: Completely At-Home Visits
The RECONNECT clinical trial will evaluate the effectiveness and safety of ZYN002, a clear cannabidiol gel applied twice a day to the skin (called transdermal application) for the treatment of behavioral symptoms of Fragile X syndrome (FXS). Eligible participants will participate in up to an 18-week study treatment period, where all participants will receive placebo or active study drug. Participants ages 3 to 29 years will be eligible to participate if they meet all entry criteria.
ZYN002, is the first and only investigational pharmaceutically produced cannabidiol, a non-psychoactive cannabinoid, formulated as a patent-protected permeation-enhanced gel for transdermal delivery through the skin and into the circulatory system. ZYN002 is an investigational treatment. This means that it is not approved by government regulatory bodies, including the United States Food and Drug Administration (FDA) and must be tested to see if it is an effective and safe study treatment.
Who can participate?
Males and females, age 3 to 29 years of age with full mutation FXS may be eligible to participate.
What will happen in the study?
- Initial Assessments to determine eligibility may be done remotely.
- If eligibility is met on these initial assessments, a visit (in-clinic or at-home) will be scheduled to complete the remaining screening assessments.
The following is a list of some of the study procedures that will happen during the study:
- Blood samples for genetic testing to confirm diagnosis of full mutation FXS and methylation status
- Blood samples and electrocardiogram (ECG) for safety monitoring
- Parent/caregiver questionnaires to assess the severity of symptoms of FXS
What are the potential beneficial outcomes that can happen from my son/daughter participating in this research?
Caregivers and their son/daughter will receive medical care associated with the conduct of the study at no charge.
Their participation will provide important information to help determine whether the study medication is safe and effective in FXS. This information will be reviewed by the FDA. If the study medication is deemed safe and effective, the medication may become available for use by other individuals with FXS.
What are the potential negative outcomes that can happen from my son/daughter participating in this research?
Overall, the active study medication has been well tolerated in previous studies in individuals with FXS. The most common adverse events are application site reactions such as pain or redness. Individuals may also experience discomfort related to the procedure to take blood samples.
There may be other risks that we do not know about yet.
Will I/my son/daughter be paid to be in this research study?
Participants will not receive payment for participation in the study. Participants may receive reimbursement for reasonable study-related costs such as travel and incidental expenses (parking, meals) and for their time in completion of some assessments. A stipend may be provided to the caregiver for completing study assessments.
Contact
To find out more, call (833) 680-1155 or visit FRAGILEXHELP.com
Interested in Participating?
If you would like to learn more about the RECONNECT trial, fill out this form and your information will be sent to the recruitment coordinator, who will reach out to you.
Our Most Recent Opportunities
FXS TECH Study
Researchers at Rush University Medical Center are working on technology to improve how to identify and track progress in children living with autism and Fragile X syndrome. The study is currently recruiting children ages 18 months to 5 years, and 12-18 years.
Study: Web Intervention for Parents of Youth with Genetic Syndromes (WINGS)
Researchers at the Autism Assessment, Research, Treatment & Services (AARTS) Center at Rush University Medical Center are currently conducting a fully-virtual research study that is testing two telehealth interventions that are designed to help parents of children with genetic syndromes and intellectual disabilities gain strategies to manage challenging behaviors.
Neural Underpinnings of the Relationship Between Cognition and Gait Dysfunction in Fragile X-Associated Tremor/Ataxia Syndrome (FXTAS)
Movement disorders researchers at Rush University Medical Center are conducting a research study to learn about brain activation in people living with FXTAS during tasks like walking and thinking. This study is currently recruiting adults ages 50+ who are living with FXTAS.
Pharmacogenomics and the Fragile X Community: Interest and Prior Understanding
Researchers at the University of Alabama are looking for members of the FX community to take their online survey so they can explore the knowledge & opinions of the FXS community on pharmacogenomic testing.
Brain & Behavior Study
Researchers at Purdue University are conducting a natural history research study to learn about brain activity in females, ages18-60 years, living with the FMR1 premutation.
NFXF Gene Therapy Community Survey
Help the NFXF - share your thoughts on gene therapy.