By Hilary Rosselot
Zynerba Pharmaceuticals Presents During the 2022 Industry Updates Keynote at the 18th International Fragile X Conference
Stephen O’Quinn, Vice President Medical Affairs at Zynerba Pharmaceuticals, presented about ZYN002, a clear, non-plant derived, cannabidiol gel, during the Industry Updates keynote session at the 18th International Fragile X Conference. ZYN002 is in Phase 3 trials in Fragile X Syndrome with the goal of becoming an FDA-approved treatment for the behavioral symptoms of FXS. Zynerba is now enrolling participants in their RECONNECT trial. More information about the RECONNECT trial can be found here.
Stephen spoke about the status Zynerba’s Fragile X program, including the results of their CONNECT-FX trial and their current RECONNECT trial, where they hope to go, and the value of partnering with families with Fragile X. Learn more about Zynerba and ZYN002 by watching their 2022 Industry Updates presentation or vising their MyFXResearch post.
about

Hilary Rosselot
Hilary joined the NFXF team in 2019. Prior to joining the NFXF team, she worked at the Cincinnati Fragile X Research and Treatment Center for over five years. She has experience as a clinical research coordinator across many types of clinical trials and served as the clinical research manager for the Cincinnati program. She earned a bachelor’s degree in psychology, a master’s, and is a SOCRA certified clinical research professional (CCRP). She enjoys time with family and friends, a great book, a strong cup of coffee and, of course, a good laugh!
learn more
Study: Autonomic and Sensory Functioning in Infants with FMR1 Conditions
Dr. Jane Roberts and the research staff at the Neurodevelopmental Disorders Laboratory at USC are conducting a research study to learn about the development of infants with Fragile X syndrome and Fragile X premutation over the first few years of life.
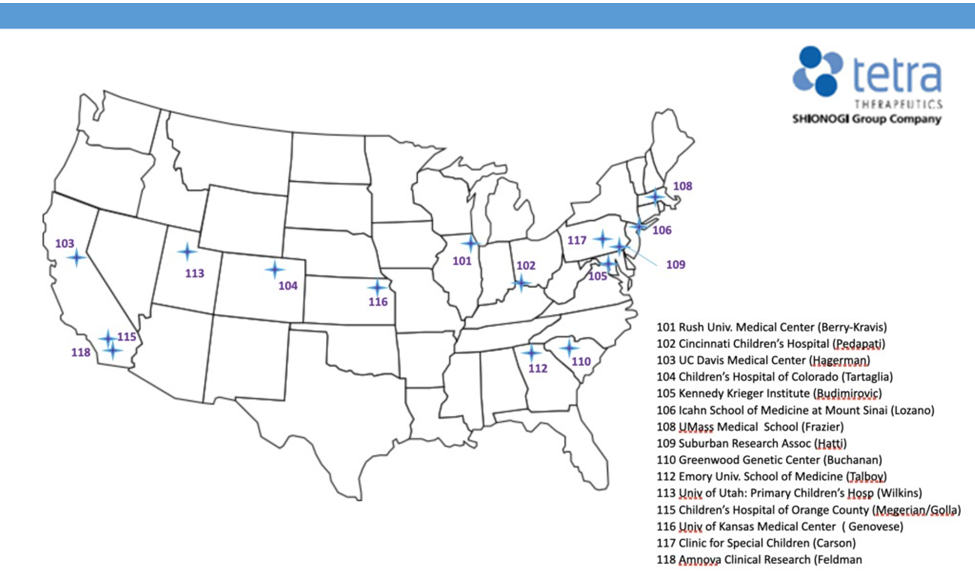
EXPERIENCE Clinical Trials in Fragile X Syndrome
Shionogi's clinical trials for males aged 9–45 living with FXS with a molecular genetic confirmation FMR1 >200 CGG repetitions testing a new drug zatolmilast.