18th International Fragile X Conference session presenters: Frank Kooy, PhD, Iryna Ethel, PhD, and Walter Kaufman, MD
Summarized by Dr. Anubhuti Goel, a 2022 Jr. Investigator

Background
While the Fragile X syndrome (FXS) field realizes that dysfunctional inhibition is the underlying cause of many symptoms associated with FXS (and likely ASD), investigating how inhibitory neurons (and by association excitatory neurons) communicate with each other will be instrumental in understanding dysfunctional sensory sensitivity, arousal, learning, social anxiety, and decision making in FXS and thus guide effective drug therapies. This is underscored by the quote from Belmonte et al, 2004, “the heterogeneity of neuropathological and genetic observations in autism suggests that autism’s essential characteristic may not be any specific cellular pathology, but rather a perturbation of the network properties that emerge when neurons interact.”
To really understand the challenges, not only at the cellular level but at the level of brain circuits, mouse models have provided a valuable characterization of the basic science that contributes to sensory, learning, and behavioral defects that resonate with human symptoms. One well-established model — the Fragile X Messenger Ribonucleoprotein 1 gene (FMR1) knockout (KO) mouse — is popular for many reasons as discussed here. The mouse Fmr1 gene product shares 97% homology with human FMRP including a conservation of the CGG repeats [2]. Fmr1 KO mice show functional alterations that are similar to humans and hypersensitivity and a few perseverative phenotypes in mice resonate with human symptoms [3, 4]. Animal models like mice allow for more granular recording methods and a broader and more nuanced toolkit of experimental manipulations.
One important idea, backed by data, that was emphasized at the NFXF meeting was the need to recognize that the different levels of FMRP protein can influence the efficacy of drugs and their impact on the diversity of symptoms. This issue directly impacts the problem of finding a reliable drug for FXS. Effective therapeutic design is further underscored by three complications: 1) an overwhelming number of cellular and molecular signaling pathways are defective in FXS. For example, one core defect that has been identified is impaired inhibition which results in aberrant communication between the excitatory cells and inhibitory cells5. There are many inhibitory cells with many downstream pathways that have diverse effects on brain function. Therefore, which pathway should be the target of drug development and testing? 2) The efficacy of a drug may not be uniform across different brain areas. 3) The current methodology of drug screening which involves a battery of electrophysiological techniques and behavior is not standardized and therefore difficult to compare across research groups.
To address these challenges, researchers in this panel described 1) novel combinatorial approaches that are being used with the help of mouse models to screen drugs and specifically test for both neuronal communication and a multitude of social behaviors, 2) Molecules that can be assayed from blood samples to provide valuable biomarkers of drug efficacy. Recent research shows that blood samples from mice yield translationally relevant biomarkers, and 3) The largely ignored influence of astrocytes on impaired inhibition in the context of FXS.
Summary of Research Highlights
Dr. Frank Kooy’s team at the University of Antwerp is working on designing a quick test for drug screening to look at neuronal activity and behavior. In recent work Dr. Kooy shows that multi-electrode arrays allow monitoring of many different neurons from a tissue in a dish, thus providing a readout of what neuronal communication is at the level of the population. This method provides a low-cost and quick assay for drug screening since the effect of drugs on neuronal activity can be easily studied by adding drugs to the medium. His recently published work shows that neuronal networks from Fmr1 KO mice show an increase in population-level network activity likely due to an increased synchronization of neurons across the network. More detailed analysis revealed that this is indeed due to an imbalance in excitation and inhibition resulting in more excitation. To access mouse behavior, his team has designed a Live mouse tracker. This allows assaying social interaction among 4 mice by tracking the mice for 24 hours monitoring individual events, social events, interactions between two or four mice, and many different parameters. This assay is versatile and can be implemented in mice of different ages, therefore useful to study the development of behavior. Both technologies are open source, allowing the same lab to assay neuronal communication and behavior for drug screening as well as allow comparison of data across labs. In the future, his team plans to combine a mouse tracker with multielectrode array (MEA) recordings in freely moving mice.
Dr. Iryna Ethell’s team at the University of California, Riverside is studying the disruptions in inhibition in FXS, but from the perspective of astrocytes. Astrocytes make up the majority of cells in the human central nervous system and influence neuronal circuits by regulating the formation, pruning, and maturation of synapses. While the impact of astrocytes has been studied in the context of glutamatergic signaling, astrocytes also regulate GABA signaling, thus impacting inhibition.
This is particularly relevant in the context of FXS. The elevated excitation described by Frank Kooy and many other research groups could be due to impaired inhibition and impaired communication between Parvalbumin (PV) and pyramidal cells7, and astrocytes could control this communication. Therefore, the main question that Dr. Ethell’s group is asking is, can astrocytes regulate this communication? While her research uses mouse models of FXS, she routinely collaborates with scientists working with humans to confirm that her research findings are translationally relevant. In one such collaboration with Dr. Maija Castren at the University of Helsinki, she found an abnormal elevation in GABA levels in human FXS astrocytes derived from patient-specific induced pluripotent stem cells (iPSCs). A similar increase in GABA was found in mouse astrocytes derived from astrocyte-specific FMR1 KO.
Further, the absence of FMRP reduced PV mRNA, percent of PV positive inhibitory synaptic sites, and excitatory innervation of PV cells. Like previous findings in human studies, EEG recordings from mice lacking FMRP in astrocytes resulted in enhanced gamma oscillations, impaired habituation to sounds, and impaired temporal synchronization of neural activity in the cortex. PV function is critical in producing timed responses; EEG recordings from astrocyte-specific Fmr1 KO mice showed that in response to sounds, cortical neurons exhibited a reduction in reliable phase-locked responses, suggesting a reduction in temporal processing, which could likely result from a reduction in PV function. Behavior in these KO mice was also impaired, resulting in hyperactivity and reduced social interactions. Therefore, in summary, Dr Ethell’s team has identified a novel locus — or place for the impaired inhibition — of astrocytes. This data adds to our understanding of the basic mechanisms that are disrupted in FXS and highlights the complication with drug screening because of the diversity in affected pathways.
Dr. Walter Kauffman from Emory University summarized recently published work from his team investigating the ameliorative (positive) effects of ANAVEX2-73 (blarcamesine) in FXS8. Blarcamesine is a Sigma-1 receptor (S1-R) agonist. Activation of S1-R leads to release of calcium, which in turn influences multiple signaling pathways and regulates key synaptic processes such as firing rate set point (e.g., hippocampus) and homeostatic synaptic plasticity. Multiple pathways downstream of calcium influx are impacted by FMRP deficiency and many of these pathways result in the production of brain derived neurotrophic factor (BDNF). BDNF plays an important role in several cognitive and sensorimotor deficits and S1-R induced changes in BDNF have been observed in many different neurological disorders. Therefore, Dr. Kauffman’s rationale is that drugs maybe be more effective if they target a common ligand that influences multiple downstream pathways. With this justification they used the Fmr1 KO mouse model to test the efficacy of Blarcamesine and found that 1) S1-R occupancy by Blarcamesine is similar in both WT and Fmr1 KO mice making it an effective drug, 2) rescued hyperactivity and associative learning, 3) improved behaviors that resonate with anxiety and perseveration observed in humans, and 4) restored levels of BDNF. Importantly, BDNF could be measured in blood samples from mice, making it a feasible biomarker of drug efficacy.
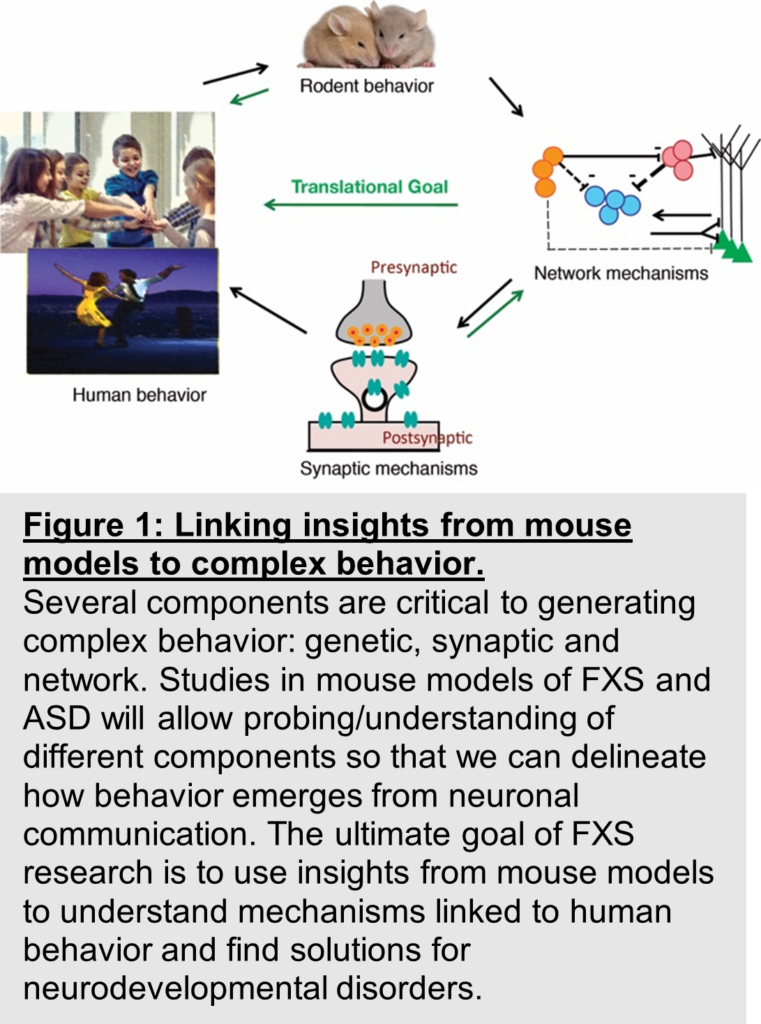
Conclusion and Insights for the future:
The field of FXS research has recognized that the path to developing viable and effective therapies in FXS needs an understanding of the relationship of impairments in synaptic and cellular physiology to atypical circuit dynamics, and how together these neuronal dysfunctions manifest in deficits in learning, social processing and other cognition impaired in FXS. Therefore, research in the field is now focused on establishing a link between basic research insights from mouse models of FXS and assays, biomarkers, and outcome measures that can be translated to solve Fragile X Syndrome in humans (Fig. 1).
Thanks to the session presenters, Frank Kooy, Iryna Ethell, and Walter Kaufmann, for sharing their research!
about

Hilary Rosselot
Hilary joined the NFXF team in 2019. Prior to joining the NFXF team, she worked at the Cincinnati Fragile X Research and Treatment Center for over five years. She has experience as a clinical research coordinator across many types of clinical trials and served as the clinical research manager for the Cincinnati program. She earned a bachelor’s degree in psychology, a master’s, and is a SOCRA certified clinical research professional (CCRP). She enjoys time with family and friends, a great book, a strong cup of coffee and, of course, a good laugh!
learn more
Every conference year, the NFXF facilitates a Jr. Investigator program. You can learn more about the program and our 2022 Jr. Investigators here:
Summary of the Research Landscape Panel at the 18th International Fragile X Conference
Dr. Kate Shelly, a 2022 Jr. Investigator, summarizes the Research Landscape panel at the NFXF's 18th International Fragile X Conference.
Summary of the Fragile X Premutation Basic Science Research Session at the NFXF 18th International Fragile X Conference
Dr. Carolyn Yrigollen summarizes the Fragile X Premutation Basic Science Research session at the NFXF's 18th International Fragile X Conference.



