Methylation
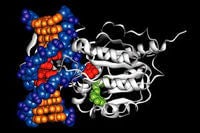 One of the ways the cells control which genetic information they will use is to chemically modify the DNA. The illustration on the right shows an enzyme (diagrammed in ribbons) adding methyl groups to some of the DNA (balls in the form of a double helix). This inactivates that part of the chromosome. It’s as if we were to put glue on the edges of some of the books in the library; those pages would become unavailable to readers.
One of the ways the cells control which genetic information they will use is to chemically modify the DNA. The illustration on the right shows an enzyme (diagrammed in ribbons) adding methyl groups to some of the DNA (balls in the form of a double helix). This inactivates that part of the chromosome. It’s as if we were to put glue on the edges of some of the books in the library; those pages would become unavailable to readers.
Good Methylation
In females, methylation is used regularly to solve a problem. Men have only one X chromosome and women have two. As a result, female cells might be expected to make twice as much protein from the information on X chromosomes as males do. Instead, women’s cells randomly pick one of the X chromosomes and turn it off by methylation. Thus both males and females have one working X chromosome in each cell and as a result, one working unit of all the genetic information on the X chromosome.
Bad Methylation
As noted above, methylation is generally a useful method for turning off chromosomal information. However, in Fragile X syndrome, methylation is involved in causing the disease. Near the FMR1 gene is a regulatory site called a CpG island. In most people, the site is not methylated. As a result, the cell can use the FMR1 gene when there is a need for FMRP—the Fragile X protein.
In people with Fragile X syndrome, the CpG island is methylated. As a result, the cell is unable to copy the information in the FMR1 gene. Since an mRNA copy is not made, FMRP will not be synthesized. Since there is no FMRP at the time and place it is needed, the characteristics of Fragile X syndrome are set in motion.
It’s Not Really the CGG’s
Much of the focus on Fragile X syndrome is on the expansion of the repeated CGG’s. It is technically not the expansion that directly causes the problem. Instead, having more than 200 CGG repeats sets in motion methylation of part of the FMR1 gene. The methylation stops the synthesis of FMRP and the absence of FMRP causes Fragile X syndrome. We do not know why having too many CGG repeats triggers methylation.
In theory, if the methylation could be removed from that spot on the FMR1 gene, it could allow access to the FMR1 gene and allow its FMRP product to be assembled. This is one of the potential treatment areas that researchers are investigating.
Mosaic Females
The inactivation of female X chromosomes by methylation, mentioned above, also seems to partially determine the impact of a full Fragile X mutation on females. Each cell in a female will inactivate or turn off one of its two X chromosomes.
If a large proportion of the cells turn off the X chromosome with the Fragile X mutation, then most of the cells will have an active X chromosome that can produce FMRP. As a result, the impact of Fragile X syndrome will be limited.
If a large proportion of the cells turn off the X chromosome with the working FMR1, then there will be few cells able to produce FMRP. As a result, the impact of Fragile X syndrome will be more pronounced.
So the severity of Fragile X syndrome on a female depends in part on whether her cells turn off mainly X chromosomes with a good FMR1 or mainly X chromosomes with a defective FMR1.

