What Is a Clinical Trial?
Includes: Orphan drug designation, FAQ
How Do Clinical Trials Work?
Includes: Discovery and development, preclinical research, clinical trial phases
How Can I Participate in Research?
Includes: Where to find opportunities
WEBINAR — Clinical Trials From Start to Finish
Includes: Where to find opportunities webinar with Sharyn Lincoln “Clinical Trials From Start to Finish”
Note: This page is a compilation of information from various authors.
Clinical research is a scientific investigation about human beings. In fact, most of what we know about treating humans is the result of clinical research. There are two main types:
- Observational: Researchers observe health outcomes, but do not assign any interventions. For example, studying the effects of a sedentary lifestyle on diabetes patients.
- Interventional: Participants receive a specific intervention, such as a drug or procedure.
An interventional study is also known as a clinical trial. Here we help you better understand clinical trials, including how they work and how to become a participant.
What is a Clinical Trial?
The FDA watches over clinical trials and creates some of the rules for how they’re run.
The FDA decides yes or no to any new medicine, and researchers must get approval from both the FDA and the designated institutional review board (also known as IRB in researcher-speak) before starting a clinical trial.
The FDA makes the final decision as to whether a new medicine can be sold in the U.S. They look at the results from all the studies and decide if the medication is safe, and if it works.
There are many ethical rules designed to protect people that are participating in research. These rules make sure that:
- Consent is voluntary, which means each participant chose and agreed on their own to be a part of the study.
- Any possible harm to participants is made as unlikely as possible.
- Researchers use the right study design to answer the questions being asked by the study.
- Participants can choose to stop participating in the research at any time.
Orphan Drug Designation
A drug is considered an “orphan” if it’s meant to treat medical conditions that are so rare (affecting fewer than 200,000 people) there is little chance of profitability for the drug developer. The term “orphan disease” refers to these rare conditions.
The FDA supports the development of new treatments for rare diseases and has the authority to grant orphan-drug designation.
What is Orphan Drug Designation? The FDA grants orphan-drug designation to a drug or biological product. This designation qualifies the sponsor for various incentives, including tax credits, exemptions from fees, and market exclusivity for seven years post-approval of the product. This is a regulatory mechanism that helps expedite drug development in rare diseases, but does not mean the product is close to clinical trials or approved by the FDA.
Frequently Asked Questions
Yes, all medications go through clinical trials, as overseen by the FDA.
A clinical trial is an important part of making a new medicine. After scientists and researchers spend many years coming up with a new drug and testing it in mice or other animals, they may get to the point of trying the drug in people. When this point is reached, it’s called a clinical trial and these trials have phases.
New Drugs: A new drug is not commercially available, which means you will not be able to have it prescribed to you after the trial is complete.
Repurposed Drugs: Some drugs are repurposed, that is, they were developed for one condition, but researchers now want to use them for a different condition.
These drugs are usually already commercially available, which means once the trial is over your clinician or doctor may be comfortable prescribing the drug for you “off label.” Off-label means you can take the drug under your doctor’s supervision, even if it is not approved for your specific condition.
If the trials are successful, researchers will work to get the condition they’re studying added to the drug label (to indicate its use as a treatment for the condition). The Food and Drug Administration must approve all changes to drug labels, and they use the results from clinical trials to make those decisions.
No! There are many other research projects that don’t involve taking a new drug. These are called “non-therapeutic studies,” and they can cover a wide range of topics. Studies that aim to develop a new questionnaire, collect medical history information, or improve quality of care would all fall under this umbrella.
The three main forms of these studies are:
- Longitudinal: The information is collected over time.
- Cross-Sectional: Many individuals participate, but data collection only happens once for each person.
- Case Study: An in-depth look at one or a couple of people.
Non-therapeutic studies are just as important as clinical trials. They help us understand more about a disorder such as Fragile X syndrome, improve clinical trials, and provide better clinical care. The FORWARD registry and database is a great example of a non-therapeutic study.
To make sure that people are respected and treated fairly, clinical research has many different rules and regulations. For example, if a patient or family is asked to join a study, they can say no. Participating in research is voluntary, which means that people must choose on their own if they would like to participate, even if it’s being run by their doctor. This is why people who join a study or trial are usually referred to as participants: it notes that they are actively choosing to participate in the research.
All studies involving human beings must be reviewed by an institutional review board. All hospitals and universities that do research use one and it’s the IRB’s job to make sure that researchers follow all the rules.
Sometimes, the IRB asks for changes to the study to make sure participants are better protected. This can make the study start later or go more slowly. Also, every group or institution that joins the study must get approval from their own IRB, which takes time.
You may hear this term if you join a trial or study.
Informed consent is the process of providing key information about a research study before a participant (you or your child) decides whether to take part.
The research team provides a document that includes details about the study that explains potential risks. You can then decide whether to sign the document or not. Taking part in a clinical trial is voluntary and you can leave the study at any time.
Researchers often need people not affected by Fragile X, which is sometimes referred to in clinical settings as “healthy” volunteers or “controls.” Healthy volunteers are needed so researchers can compare results between people with and without a certain disease or condition. Once you find a study you might be interested in, contact the study team! They will be able to provide you with more details about the study.
How Do Clinical Trials Work?
The drug development process from start to (hopefully) an FDA-approval can take over 12 years and $1 billion. Here we discuss clinical trials as they relate to Fragile X.
Discovery and Development
Drug development starts with discovery and development. Many compounds — possible future drugs — are tested and usually only a few look promising enough to move forward with development.
Preclinical Research
Before testing in humans, researchers must determine whether the drug could cause serious harm or toxicity.
Preclinical research is also important because developers strive for a good translation of results from their preclinical Fragile X syndrome model to humans with Fragile X syndrome. Preclinical research is important because it helps researchers understand how the drug may impact humans and make sure it is safe.
There are mouse, rat, and even fish and fly Fragile X syndrome models. Some groups also use induced pluripotent stem cells, known as iPSCs. These are a type of pluripotent stem cell that can be generated directly from a somatic cell, like a blood or skin cell. (Somatic cells are any cells other than reproductive — sperm and egg — cells. They contain a full set of chromosomes vs. only half in reproductive cells.)
- Preclinical research tests the compound in the Fragile X syndrome model and collects data on:
- How it’s absorbed, metabolized (processed or used by your body), and excreted (removed from your body)
- How it interacts with other drugs
- How to best administer it
- Any potential side effects and benefits
- Potential dosing ranges
Animal Model: Mice, frog, and even fish and fly models have been used in Fragile X research. What is an animal model?
An animal model is a non-human species used in medical research because it can mimic aspects of a disease found in humans. Animal models are used to obtain information about a disease and its prevention, diagnosis, and treatment. By using animals, researchers can carry out experiments that would be impractical or ethically prohibited with humans. (Source: National Human Genome Research Institute genetics glossary↗)
Investigational New Drug Application: At the conclusion of preclinical research, and before beginning clinical research, an investigational new drug application is filed with the FDA. Known as an IND, the application includes drug information, preclinical research data, and plans for future clinical trials.
In response, the FDA provides guidance that may help enhance the developer’s clinical trials and, if approved, gives official permission to start research in humans, and phase I can begin.
ARE YOU A RESEARCHER? We — the NFXF — like to initiate an ongoing relationship with drug developers that continues all the way through the clinical trials to bringing the drug to market. We believe these relationships are most successful when they begin before or during the early preclinical phase.
Please review our NFXF Research Readiness Program, and we look forward to hearing from you.
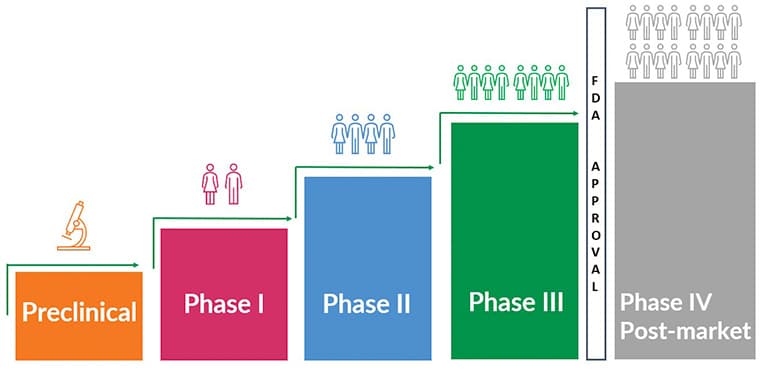
Clinical Trial Phases
There are three main phases in a clinical trial — and sometimes there is a fourth, or post-market, phase that studies and assesses the long-term safety and effectiveness of the drug. This phase only occurs if the drug is approved by FDA. And sometimes there are in-between phases, for example, Phase 1a or a Phase 2b. This is especially common in rare disease research such as Fragile X.
How Can I Participate in Research?
Some studies are very easy to do — and short! Many, such as surveys, can be done at home. Other studies, especially clinical trials, can come with a big time commitment. COVID-19 pushed many researchers to be flexible about how they conduct research. This means there may be even more accessible research options for you to consider.
We’ve listed some resources for finding research opportunities below. If you do decide to move forward, here are four important things you can ask about, to make sure that the study is right for you:
Will you or your child take the study medication, or is there a “placebo” (fake drug) involved? Are there forms you need to complete on a regular basis?
It can be scary to think about getting a placebo in a drug trial. Comparing the drug to a placebo is an important way for the researchers to gather data on the drug. If you are concerned about placebo, ask the study doctor to explain more about why it is important to help you make your decision.
One day we hope that there will be synthetic placebo arms, which are placebo arms for trials that are made by using data from other research projects. It takes a long time to build up enough data to do this. The NFXF Data Repository is working on making this possibility a reality.
It’s important to make sure that completing all the study activities — which can include phone calls, daily diaries, online forms, and visits to the study site — is feasible for your family.
Find this out and make sure you have enough time in your schedule for all the study activities.
For clinical trials and many other studies, there can be a list of medications or other factors that limit — exclude — who can participate. Ask the study team about any limits on who can join the study.
Exclusion criteria sometimes includes things like sex, gender, or the ability to speak. This can be very frustrating and feel like you’re being excluded. We know that we will need more than one medication to treat all the different individuals with Fragile X, so why exclude anyone? Researchers may have strict exclusion criteria because their prior research on the drug shows it works for a specific group of people and they want to test that specific group to see if they’re correct. If they are correct, it’s easier for the researcher to broaden the scope, adding more people to a future trial that may have previously been excluded.
If so, how will they be communicated to me?
The research doesn’t end as soon as you’ve done all the study activities! Once the last person has completed the study, all the data needs to be analyzed. In other words, all the data will be looked at to see if there are any patterns that help lead to an answer to the questions being asked in the study. This can take a lot of time and is another reason why it can take so long to get results from a study. You can always contact the study team to see if new information is available.
Most of this information should be part of the initial description of the research opportunity, but if not, be sure to find out everything you can. Remember, you’re the expert on you and your family! Share your concerns, confusions, and questions with the study team as you talk about the project.
- What is the target of the medication or treatment?
- Will there be any therapy interventions used along with the medication?
- What kind of tests will be done before and after the medication?
- Is blood work required? How often?
- Is it for males, females, or both? What are the ages?
- Is it okay to be on other (existing) medications?
- Do we need to travel? Where?
- How many visits are required? How long do they take? Are they flexible?
- What do I have to do at home, outside of the study visit?
- Does the trial pay for travel costs, such as airfare, hotel, or childcare?
- Is there a placebo aspect to the trial?
- Can taking the medication continue after the trial ends?
- Which condition are you looking for, and what about mosaicism?
- Is it only for individuals in the U.S.?
- Please explain why to get involved.
- Who do I contact if I have questions during the trial?
- Will there be a detailed schedule for each visit?
Where to Find Opportunities
The NFXF keeps an up-to-date searchable database called the MyFXResearch portal for research opportunities involving Fragile X syndrome and related disorders (including non-patients).
Here are some other great places to look:
- Clinicaltrials.gov: All medication trials (and some other studies) have to register here, so you can get all the latest information on the study: Who is sponsoring it, where it’s taking place, even some preliminary results. You can search for “Fragile X Syndrome,” or for any other condition you or a family member might have (including FXPOI and FXTAS).
- Local and national groups like the NFXF’s Community Support Network.
- Your local clinic! Clinicians and researchers at your nearest Fragile X clinic may already be working on projects that you can help with.
- Research registries: The study teams will contact you if you or your child appears they might be eligible for a study. One example is the FORWARD study, taking place at a Fragile X Clinic near you! The International Fragile X Premutation Registry is another great example. This Registry is for adults who are Fragile X premutation carriers and their family members.
Watch Clinical Trials From Start to Finish
Sharyn Lincoln and Katherine Pawlowski of Boston Children’s Hospital speak with us about how clinical research trials work, what it takes to participate, and what happens after the study. Note that this is from 2017 and some things within the Fragile X research landscape may have changed (as they always do).
about

Hilary Rosselot
Hilary joined the NFXF team in 2019. Prior to joining the NFXF team, she worked at the Cincinnati Fragile X Research and Treatment Center for over five years. She has experience as a clinical research coordinator across many types of clinical trials and served as the clinical research manager for the Cincinnati program. She earned a bachelor’s degree in psychology, a master’s, and is a SOCRA certified clinical research professional (CCRP). She enjoys time with family and friends, a great book, a strong cup of coffee and, of course, a good laugh!
author

Sharyn Lincoln, MS, CGC
Sharyn is a licensed genetic counselor and program coordinator of the Fragile X Program at Boston Children’s Hospital. In her spare time, Sharyn is auntie to the cutest Bernese Mountain Dog ever.
author

Katherine Pawlowski, BA
Katherine is the research manager of the Fragile X Program at Boston Children’s Hospital. In her free time, she enjoys taste-testing baked goods and reading lots of mystery novels.
learn more
Best Practices in Fragile X Syndrome Treatment Development
In this peer-reviewed article, the authors review key aspects of the process of FXS treatment development with an eye toward ensuring that successful trials of new treatments, incorporating innovative research and stakeholder concerns, can be enacted.
Learn About Clinical Studies↗
From ClinicalTrials.gov, maintained by the National Library of Medicine (NLM) at the National Institutes of Health (NIH).
Zynerba Shares their RECONNECT trial
Zynerba presented about their prior results and their current RECONNECT during the 2022 Industry Updates keynote session at the 18th International Fragile X Conference.
Healx Shares their IMPACT-FXS trial
Healx Ltd presented about the IMPACT-FXS trial during the 2022 Industry Updates keynote session at the 18th International Fragile X Conference.