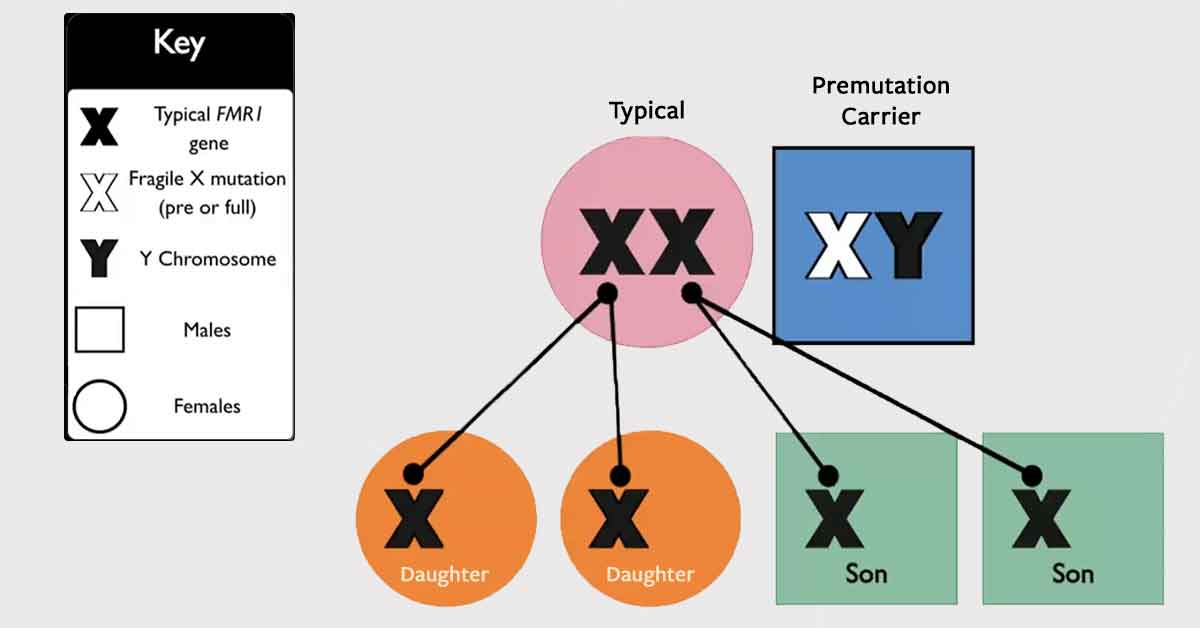
Gene Therapy Community Survey Results
Presented by Anna De Sonia (5 min.). To better understand the FX community’s thoughts, feelings, and attitudes towards gene therapy, the NFXF launched a mini-survey earlier this summer.
Over 350 people completed the mini survey and shared their hopes, concerns, and how much they already know (or don’t know) about gene therapy.
High-level results from this community survey:
- Most people had some previous knowledge about gene therapy before taking the survey.
- Most people were unaware of the different types of gene (or ”genomic”) therapies available.
- When asked what people want to know most about gene therapy, results showed the most popular responses were:
- How does gene therapy work?
- When will gene therapy be available in FXS?
- How long do gene therapy effects last? Is this a cure?
- Top concerns about gene therapy included:
- Side effects & risks.
- Timeline (when will this be available in FXS?).
- Treatment frequency (how many times ).
- Effectiveness (is this a cure?).
- 95% of survey takers said they would consider gene therapy for themselves/their loved ones living with FXS
Gene Therapy Survey Results Q&A
Presented by Hilary Rosselot (48 minutes). Hilary Rosselot, executive director at the NFXF, moderated a brief Q&A and panel discussion following the presentation of the gene therapy survey results.
Experts in the field shared their own opinions and feelings about gene therapy and what they’re most excited about for the future as we continue to make strides in Fragile X research.
Hilary kicked the panel discussion off by exploring the language and terms used when talking about “gene therapy”:
- Dr. Elizabeth-Berry-Kravis pointed out that “gene therapy” technically refers to transferring a gene back into the body. Most of us think ”gene therapy” is all-encompassing, but it actually refers only to this one avenue of treating someone’s genetics.
- The most common form of ”gene therapy” is through a viral vector. You can take the gene, put it into a virus and give it to the affected person so the virus can transfer the gene into the cells of interest.
- There are other ways to treat genetic conditions, though, and Dr. Berry-Kravis calls these ”genetically targeted strategies.” These work differently than introducing a gene back into the body. These include:
- ASOs – where you introduce a small piece of DNA that goes and binds to RNA and changes your genetics (i.e. what your genes are doing).
- Other strategies that are currently in development (but still far from being implemented in humans) such as “editing” a gene. This could look like removing the “bad” part of the gene or changing a gene to have a “normal” gene sequence.
- Reactivation – where you “turn on” part of the gene that’s not working.
- Dr. Rey Lozano and Dr. Randi Hagerman further elaborated on genetically targeted strategies utilizing protein.
- Dr. Peter Todd added that he prefers the term “genomic therapeutics” to encompass all types of genetic treatment avenues. We like this term too!
When asked about how genomic therapeutics will be given to people, Dr. Hagerman explained that it really depends on the type of therapy:
- Protein replacement therapies may use an IV in the arm.
- Gene therapies (virus carrying “normal” gene) are injected directly into the spinal fluid so the gene therapy can go right into the brain.
- Gene therapy is a “one and done” situation since bodies will develop a response to the virus.> ASOs use injection into the spinal fluid on a timed schedule (example: injection every 3 weeks).
- It is still unknown how future therapies will be delivered into humans.
- Dr. Rey Lozano pointed out that these trials are not yet available in FXS.
- Dr. Peter Todd added that while these technologies are not being developed specifically for FXS, the development of these technologies in common conditions will drive the delivery method, which will then be adaptable to FXS.
Experts discussed the topic of safety and development of genomic therapeutics, touching on topics like “How do we know that these therapies are safe?” and “Where do these therapies start being developed and how do we know that they will be safe in humans?” This led into discussion about how some therapies are reversable and some aren’t, and potential side-effects.
Panelists discussed the question of “who will be eligible?” when these genomic therapeutics eventually arrive into the FXS space. Experts agreed that while these therapeutics will help adults, they may help children more since the brain is still developing in children. However, researchers must play within the bounds set by the FDA and have to follow their drug-development process. This doesn’t mean that researchers won’t push to make this available to children earlier on though!
Hilary presented an important hypothetical situation: ‘If I participate in one genomic therapy trial, am I eligible to participate in another one?’ and experts all had the same response — put simply — no. While drugs/therapies are under investigation, people in trials are only allowed to participate in one at a time, and participating in some trials may exclude you from participating in future trials.
- This is why it’s so important to do your research, feel all the feelings, talk to your experts, and ask all the questions before deciding if a genomic therapy is right for you.
The panelists were presented with a really interesting and important question: “Do genomic therapies change the core of who you are?” Dr. Berry-Kravis, who has administered ASO therapies in Angelman syndrome, another developmental disorder, shared that she hasn’t noticed any changes to the personalities of her patients who have received these treatments. Dr. Todd added that we can’t ever really know, until later, who people will become, and reminded us that “change, for many of us, is good.”
The panel wrapped up by describing what they’re looking forward to most in terms of genomic therapeutic advancements in the FX field:
- Dr. Lozano pointed out that gene therapy has been around for decades but the recent advancements to the field make gene therapy a strong possibility for the near future.
- Dr. Hagerman emphasized that genomic therapies will be remarkable for many disorders, including FXS. She stated that while genomic therapy is “not for everyone…for many, it will absolutely change their lives.”
- Dr. Berry-Kravis said she’s looking forward to “cocktails” in a play on words. In this case, she meant that it’s likely that nothing is going to work perfectly on its own, but genomic therapies combined together could provide a “massive improvement”. Oh, and she’s looking forward to getting approval so she and Dr. Hagerman can retire.
- Dr. Todd said he’s looking forward to actual cocktails at the retirement party of Liz & Randi after finally getting FDA approval. He finished by stating that his career won’t be complete until a cure is found.
Anna said she’s most excited to see the impact of genomic therapies on the community as a whole — the “big picture.” Who knows how great it will look!
The NFXF has compiled a group of experts into a gene therapy committee. This committee will help the NFXF create content about genomic therapies that will be shared with you, so you can stay informed. Keep a look out for more genomic therapy content in the near future!