February is Rare Disease Month and a great time to consider whether your loved one is eligible to participate in a Fragile X syndrome clinical research program.
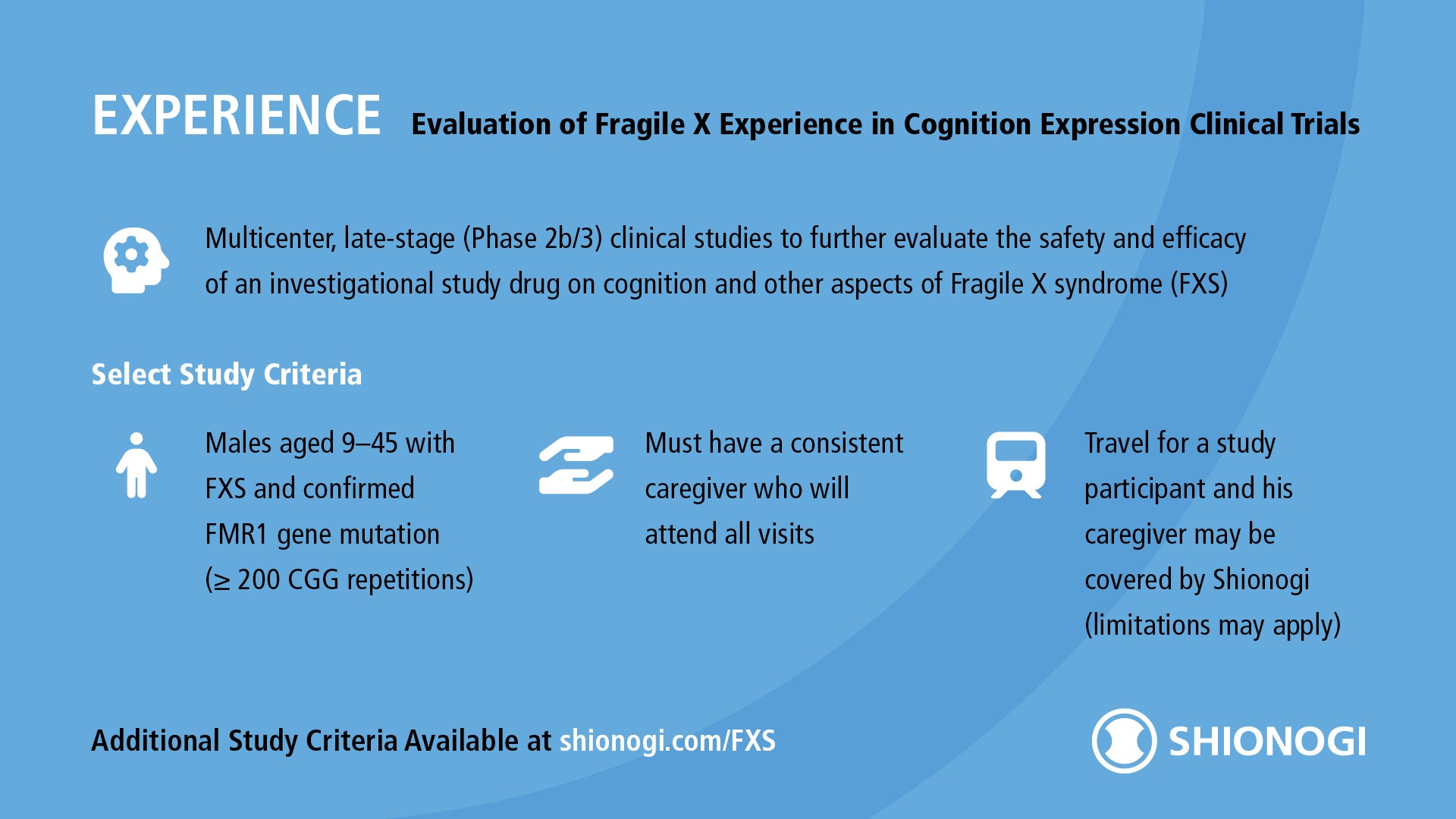
The EXPERIENCE clinical studies, Shionogi’s ongoing Phase 2b/3 studies for an investigational drug for Fragile X syndrome, are still enrolling qualified male participants aged 9-45 with FXS and confirmed FMR1 gene mutation (≥ 200 CGG repetitions) at sites across the U.S.
You may have heard about EXPERIENCE (Evaluation of Fragile X Experience in Cognition Expression) clinical trials as the Tetra studies or the studies of BPN14770 in Fragile X syndrome. The EXPERIENCE clinical studies are now being managed by Shionogi (the company that acquired Tetra Therapeutics Inc. in 2020).
Travel to and from sites for a study participant and his caregiver may be covered, and may also include transportation, lodging arrangements, and reimbursement for meals (limitations may apply).
Find out about EXPERIENCE on our EXPERIENCE research opportunity or on the Shionogi website Fragile X Syndrome Clinical Trials. You can also contact Shionogi directly at medinfo@shionogi.com or 1-800-849-9707.