Research Results Roundup
We are excited to share with you some of the great research results that have been published thus far. We are committed to keeping the community up to date on research findings, taking time to reflect and celebrate successes in the field of Fragile X-associated conditions and disorders.
Researchers: If you’ve recently published your scientific findings in a peer-reviewed journal, please share your work with us by emailing research@fragilex.org.
Cheers to the amazing scientific advancements in our field and the advancements to come. It would not be possible without your dedication, passion, and drive to make each day just a bit better.
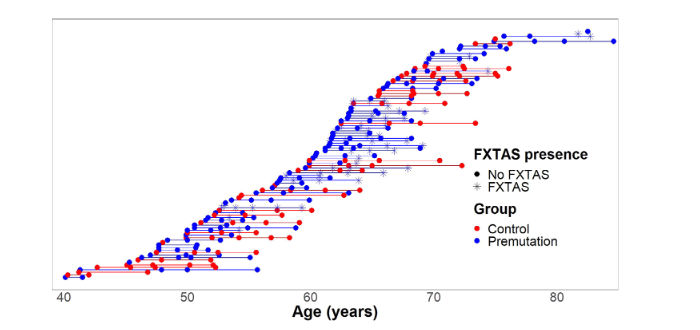
FMR1 Carriers Report Executive Function Changes Prior to Fragile X-Associated Tremor/Ataxia Syndrome: A Longitudinal Study
Authors: David Hessl, PhD, Karina Mandujano Rojas, BS, Emilio Ferrer, PhD, Glenda Espinal, BS, Jessica Famula, MS, Andrea Schneider, PhD, Randi Hagerman, MD, Flora Tassone, PhD, and Susan M. Rivera, PhD Summary: People with [...]
Healthcare Experiences of African American Women with a Fragile X Premutation
Authors: Andy King, Nadia Ali, Cecelia Bellcross, Fabienne Ehivet, Heather Hipp, Jessica Vaughn, Emily G. Allen An estimated 1 in 291 women carry a Fragile X premutation (PM) and there is little evidence that [...]
Emotion Dysregulation in Fragile X Syndrome
By Mya Jones Authors: Rebecca C Shaffer, Debra L Reisinger, Lauren M Schmitt, Martine Lamy, Kelli C Dominick, Elizabeth G Smith, Marika C Coffman, Anna J Esbensen Summary: A large portion of individuals with Fragile [...]
Antisense Oligonucleotide Rescue of CGG Expansion–Dependent FMR1 Mis-Splicing in Fragile X Syndrome Restores FMRP
One of the most exciting advancements being done in Fragile XS research today is antisense oligonucleotide (ASO) therapy.
The effect of college degree attainment on neurodegenerative symptoms in genetically at-risk women
Researchers at the University of Wisconsin explored the relationship between obtaining a college degree and the manifestation of the neurodegenerative symptoms of FXTAS among women at elevated genetic risk.
Fragile X-associated tremor/ataxia syndrome rating scale: Revision and content validity using a mixed method approach
Researchers across several institutions set out to develop a revised version of the FXTAS-RS designed to specifically assess FXTAS motor signs.
FMR1 CGG Repeats and Stress Influence Self-Reported Cognitive Functioning in Mothers
Researchers at the University of Wisconsin looked at the relationship and influence of FMR1 CGG repeats and stress on self-reported cognitive functioning in mothers.
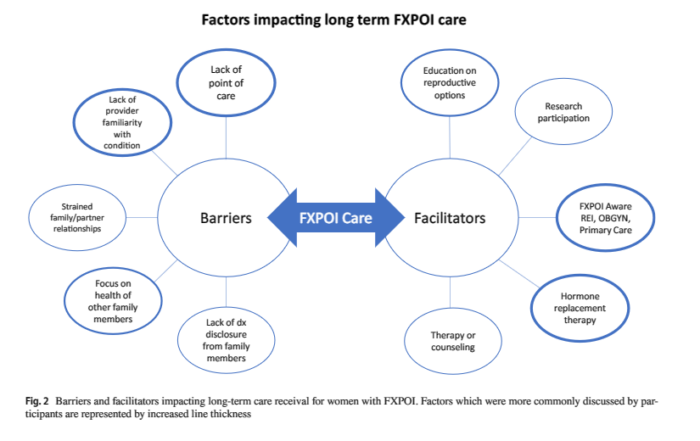
The diagnostic experience of women with fragile X–associated primary ovarian insufficiency (FXPOI)
Researchers at Emory University conducted qualitative interviews with 24 women with FXPOI exploring how FMR1 screening, physician education, and supportive care impacted their experience receiving a diagnosis. Their results are in!
Questions?
If you have questions about anything research-related, we’d love to hear from you! You can reach out to Hilary Rosselot directly, or submit your question or comment through our contact form below.

Hilary Rosselot, Director of Research Facilitation
hilary@fragilex.org | (202) 747-6208
Last Updated: 06/04/2021