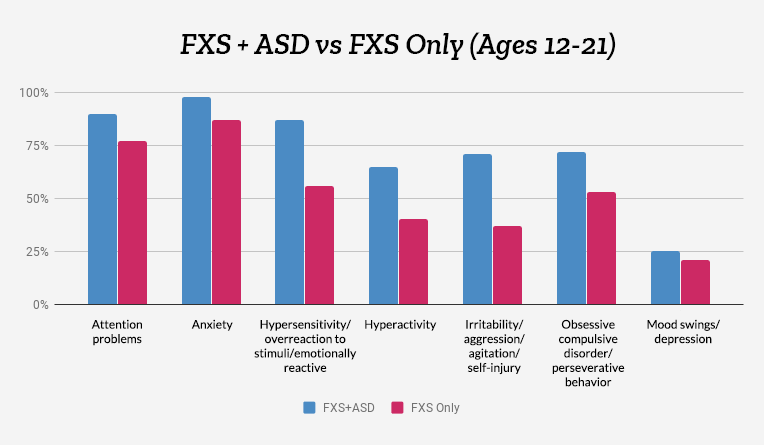A Message from Harmony Biosciences about the RECONNECT Trial and the conclusion of its Open Label Extension (OLE) program
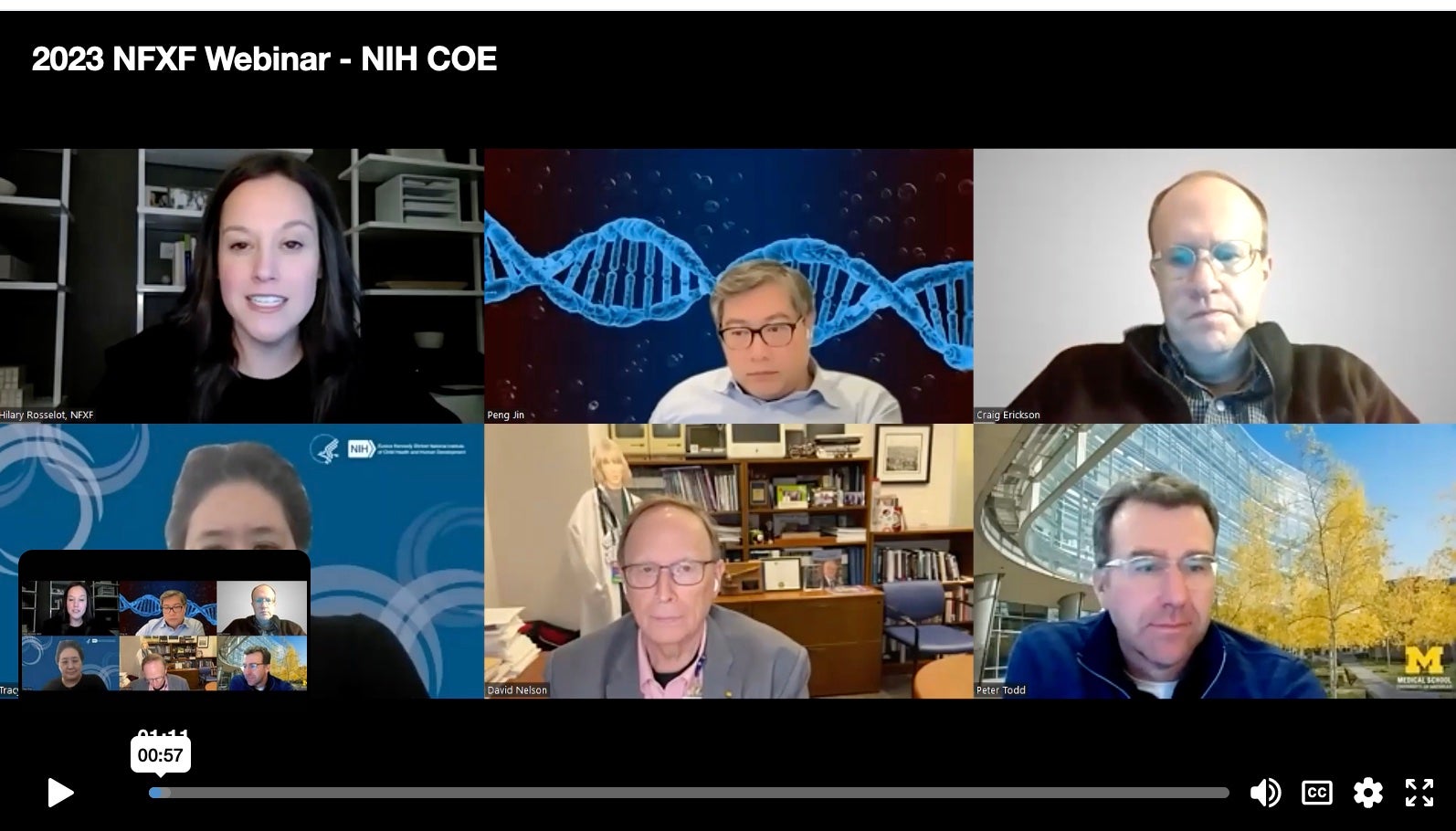
In the first webinar of NFXF’s 2026 Webinar Series, we hear updates from each of the current NIH-funded Fragile X Centers of Excellence. This funding remains critical to sustaining progress and advancing future discoveries.
The NFXF hosted an informative webinar featuring Dr. Elizabeth Berry-Kravis, who shared updates on the CDC-funded FORWARD-MARCH study and new insights into Fragile X syndrome and premutation health. The study builds on more than a decade of CDC-supported FORWARD Fragile X research, which began in 2012 and continues to collect nationwide data to advance understanding of Fragile X syndrome.
Dr. David Hessl joined the NFXF to discuss the exciting new ACT for FXTAS study, funded by the National Institute of Neurological Disorders and Stroke (NINDS).
Mirum Pharmaceuticals has officially enrolled the first participant in its BLOOM Phase 2 clinical study evaluating MRM-3379, a potential new treatment for Fragile X syndrome (FXS).
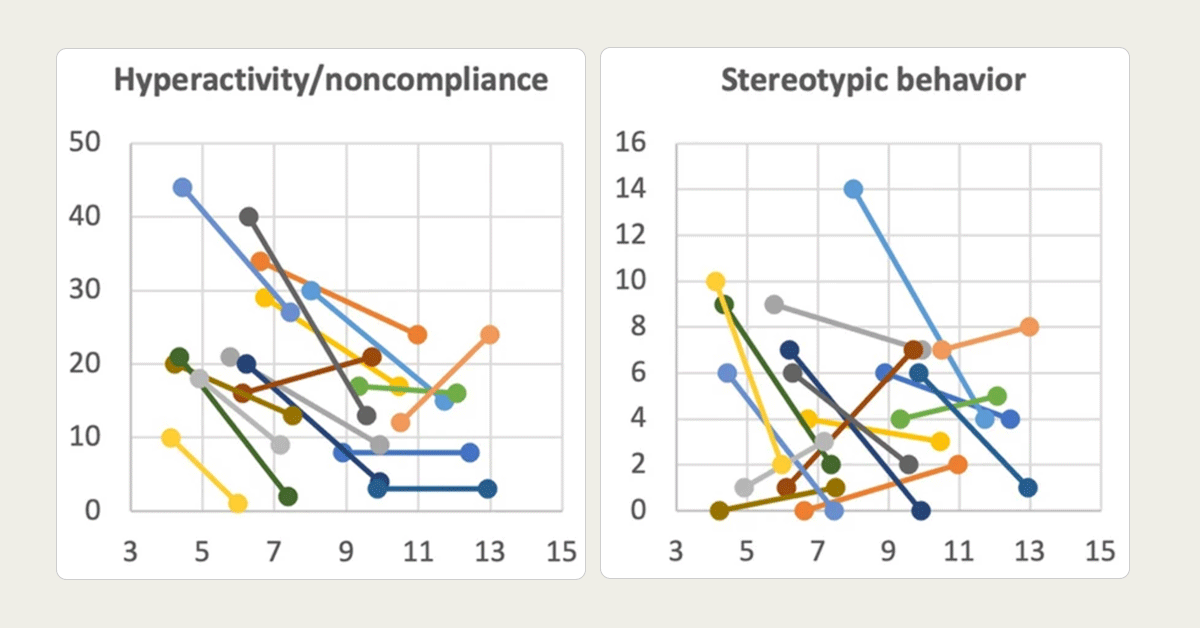
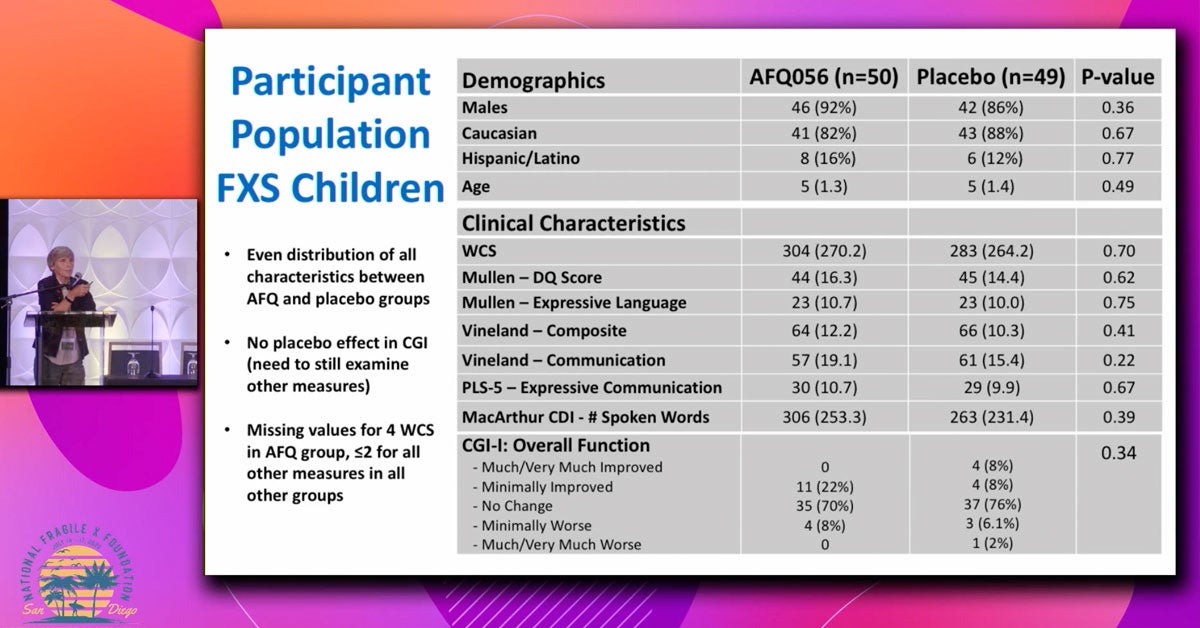
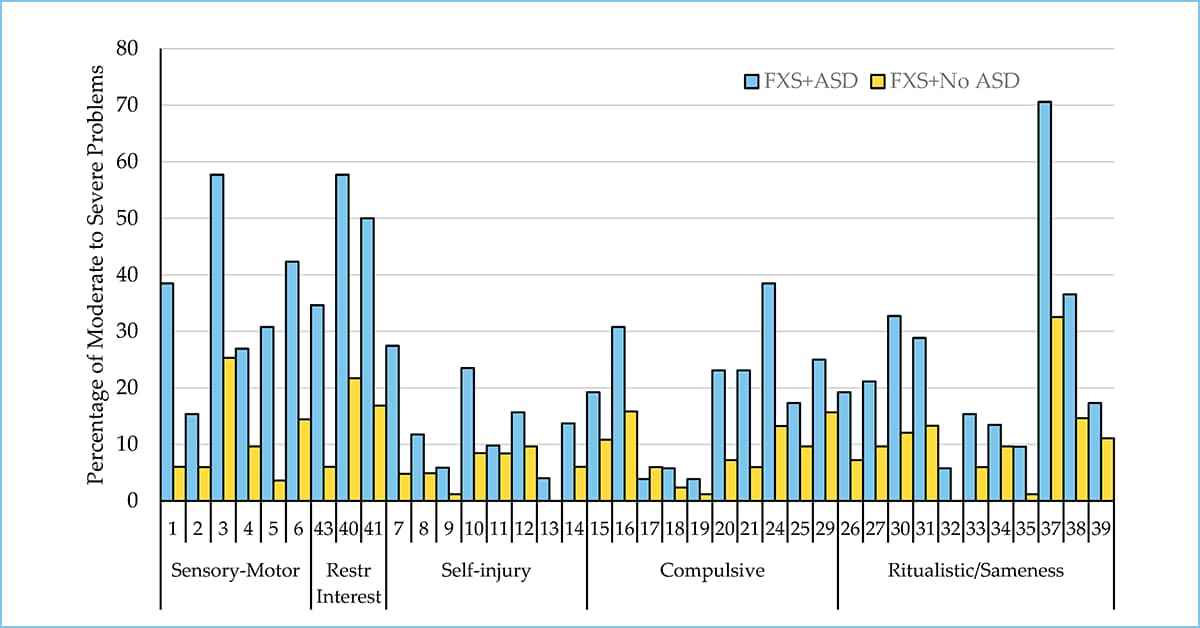
This research looks at how a child’s level of intellectual disability (ID) — meaning how much their thinking and learning abilities are affected — relates to the types of behavioral challenges they experience if they have Fragile X syndrome (FXS).
Four of our industry partners developing treatments for Fragile X syndrome — Shionogi, Mirum Pharma, Kaerus Bioscience, and Servier Pharmaceuticals — shared their latest research updates during a live webinar on October 28, 2025.
CONNECTA Therapeutics is taking a new approach to Fragile X syndrome treatment by boosting the brain’s natural ability to reconnect and adapt.
Harmony Biosciences recently announced results from its Phase 3 RECONNECT study of ZYN002, a cannabidiol (CBD) gel being tested for Fragile X syndrome.
Bowen’s FX Therapeutics, a company with roots in the Fragile X community, is advancing efforts toward a future protein replacement therapy.
On July 27, 2024, at the 19th NFXF International Fragile X Conference, three of our industry partners working on treatments for Fragile X syndrome presented updates on their research.
We’re on a mission to enroll 600 participants with FXS born in 2003 through 2020. We’re halfway there and we need your help!
In our first webinar in the NFXF’s 2025 Webinar Series, we hear updates from each of the current NIH-funded Fragile X Centers of Excellence. Why is this year’s Advocacy Day especially important? Congress is gearing up to make crucial decisions about our nation’s priorities and how to fund them. The NIH is one of those federal funding bodies supporting Fragile X research for over two decades.
The Belonging Project aims to intentionally extend our reach to underserved and underrepresented communities across the United States. We have 2025 updates from three Fragile X clinics and our belongingness survey, and we will keep you updated as we continue to move forward.
Get the latest insights on basic and translational science research in Fragile X syndrome from these 19th NFXF International Fragile X conference sessions.
Get the latest insights on clinical research related to the Fragile X Premutation from these 19th NFXF International Fragile X conference sessions.
Get the latest insights on basic science research related to the Fragile X Premutation from these 19th NFXF International Fragile X conference sessions.
In this keynote session at the 19th NFXF International Fragile X Conference, Dr. Peter Todd discusses the history, challenges, and advancements that have helped shape the trajectory of treatments for Fragile X-associated conditions.
Get the latest insights on clinical science research in Fragile X syndrome from these 19th NFXF International Fragile X conference sessions.
Get the latest insights from the FORWARD-MARCH Fragile X study on adult life, medical issues, sensory symptoms, and behavioral subtypes in Fragile X syndrome.
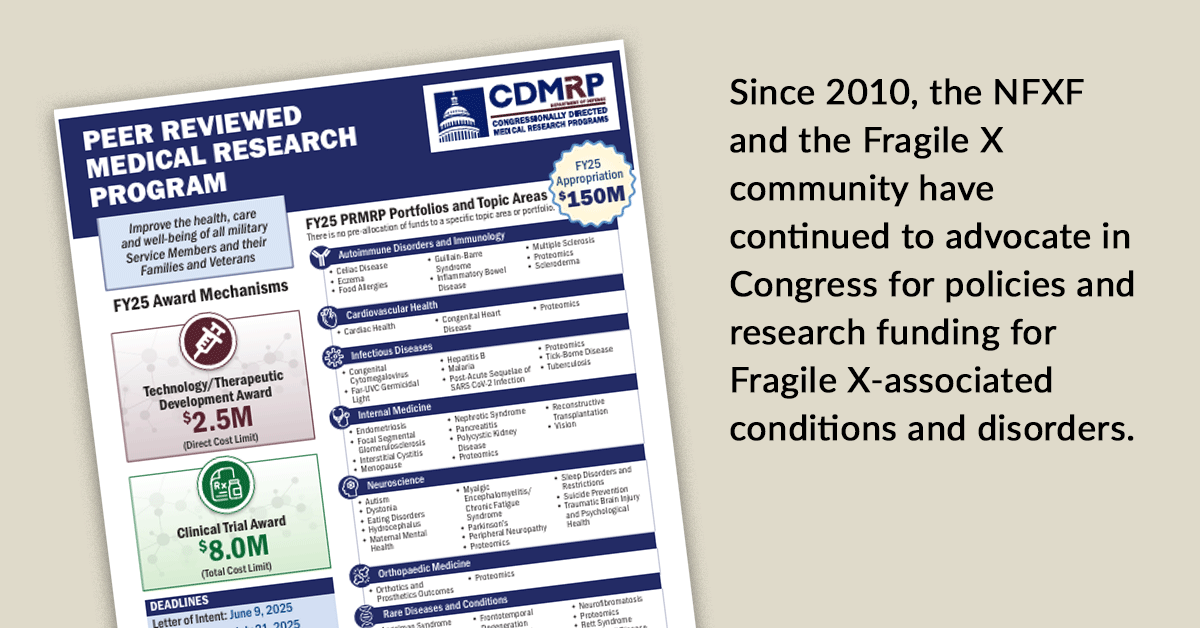
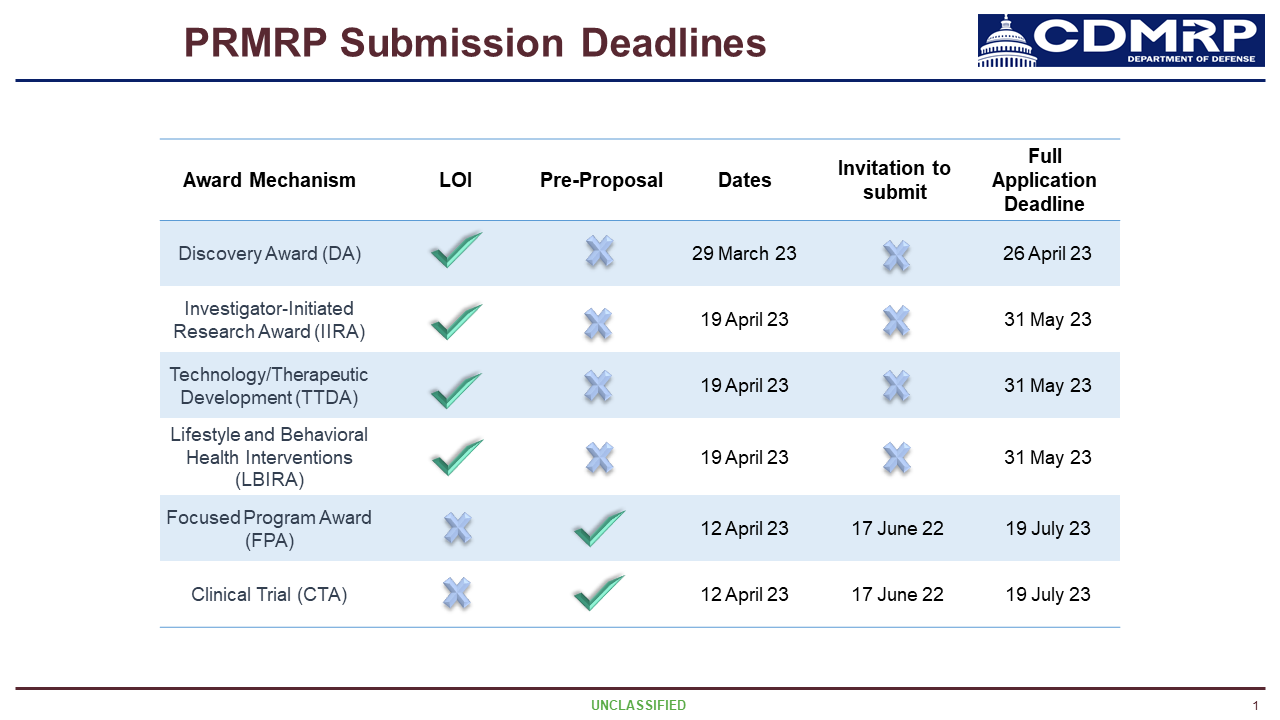
Dr. Argue shares information reviewing the details of the Congressionally Directed Medical Research Program, specifically the Peer-Reviewed Medical Research Program. Thanks to the efforts of NFXF advocates, Fragile X, which includes all FMR1-associated conditions and disorders, is once again included as an eligible topic area for FY 2025.
Healx presents during the 2022 Industry Updates Keynote at the 18th International Fragile X Conference.
Dr. Elizabeth Berry-Kravis and Dr. Walter Kaufmann presented two of the “most-cited” publications from the American Journal of Medical Genetics Part A and answered questions during a moderated Q&A.
Announcing FY 2025 federal research funding opportunities across two award categories available for all Fragile X-associated conditions and disorders.
FXTAS was first described in five grandfathers of children with Fragile X syndrome in 2001 by Dr. Randi Hagerman and her colleagues.
Harmony Biosciences shares that they have reached their enrollment goal for the Phase 3 RECONNECT trial and screening into the trial has officially closed.
A message about Shionogi’s EXPERIENCE clinical trials from Dr. Juan-Carlos Gomez, Chief Medical Officer at Shionogi & Co., Ltd.
The National Institute of Health (NIH) recently released a Request for Applications (RFA) for research in Fragile X syndrome (FXS) and FMR1-associated conditions.
You Spoke, We Listened: The Completely At Home RECONNECT Clinical Trial –Participate in Research Without the Stress of Traveling
You may have heard about EXPERIENCE (Evaluation of Fragile X Experience in Cognition Expression) clinical trials as the Tetra studies or the studies of BPN14770 in Fragile X syndrome. EXPERIENCE is now being managed by Shionogi and clinical trial sites across the U.S. are still enrolling qualified male participants aged 9-45.
We live in a day and age of social sharing, and that is not going anywhere. However, when we participate in a clinical trial, we have to understand that we cannot share everything in order to protect the integrity of the trial.
One of our newest initiatives aims to intentionally extend our reach to underserved communities across the United States. In partnership with four Fragile X clinics, we’ve begun work to understand the challenges to diagnosis and treatment faced by Black, Hispanic and Native American communities and the providers who serve them.
In the latest from the NFXF Webinar Series, panelists discuss the advances made in understanding Fragile X Syndrome through FORWARD, the Fragile X Online Registry With Accessible Research Database.
The Congressionally Directed Medical Research Program could face a 57% reduction in funding with the proposed continuing resolution.
Kaerus Bioscience has completed its Phase 1 trial with its novel BK channel modulator, KER-0193, being developed for Fragile X Syndrome!
This report, from Summer Scholar Marwa Zafarullah, is based on a study to help us better understand the impact of the CGG repeats on the different clinical premutation phenotypes and the molecular mechanisms behind the development of Fragile X-associated disorders.
Research Summary: This research was very revealing. Currently, we do not have accurate methods to predict who will develop FXTAS and who will not, or when the disease will start.
Dr. Argue shares programmatic details, deadlines, and tips to support researchers throughout the application process, and answered questions from attendees.
During this 45-minute Lunch & Learn webinar, Dr. Erickson discussed details of a single-dose medication study in Fragile X syndrome (FXS), including how the study was done, what the results showed, and moreover, why the results are important, what they mean for the FXS community, and next steps to move us forward.
In the second webinar in the NFXF’s 2024 Webinar Series, Dr. Tracy King and representatives from each of the current NIH-funded Fragile X Centers of Excellence shared updates and answered questions about their progress to date.
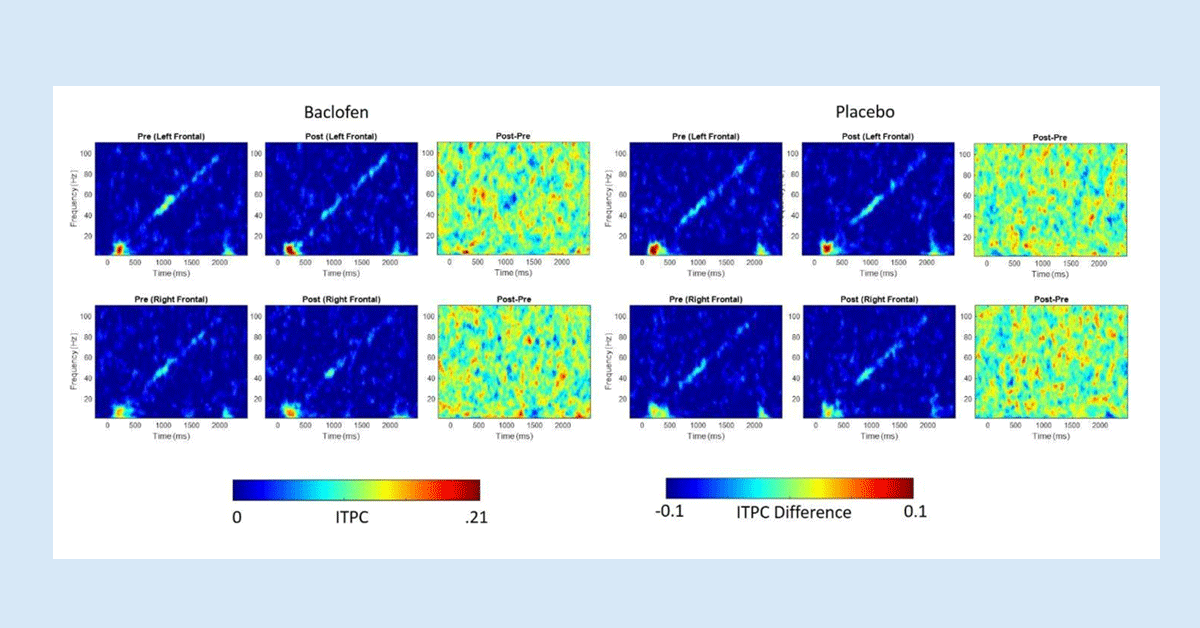
Research Summary: The results found from the EEGs in both the FMR1 KO mice and humans with FXS are extremely important for the future of FXS research because they show a measurable commonality between the two very different species.
Over the past 15 years, CDC has funded four FORWARD (Fragile X Online Registry With Accessible Research Database) Fragile X studies to expand understanding of Fragile X syndrome (FXS).
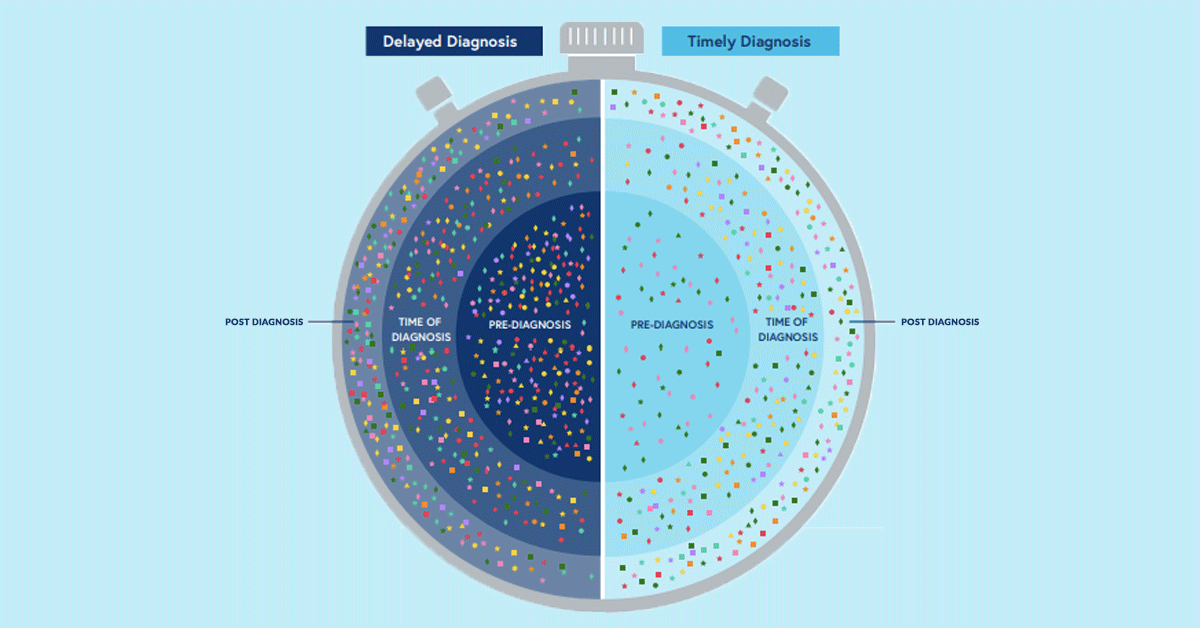
We are proud to be part of the Everylife Foundation’s newest report, which uses real-world data to evaluate healthcare usage in seven rare diseases and its relationship to timely diagnosis and the diagnostic odyssey.
On September 19, 2023, the NFXF hosted a Fragile X research update webinar with three of our industry partners working on treatments for Fragile X syndrome.
PureTech Health has been awarded a grant from the DOD for their trial of LYT-300, oral formulation of allopregnanolone, in people with FXTAS.
In this three-minute video, Dr. Liz Berry-Kravis shares what participants are asked to do during the trial, who is eligible, and how to learn more.
Research Result: Overall, the study results suggest a possible treatment to restore FMRP in individuals with FXS. These findings also suggest that abnormal RNA sequencing events identified in white blood cells may serve as powerful biomarkers for FXS.
More research is needed to better understand the progression of FMRP expression across the lifespan and how FMRP is related to all aspects of functioning.
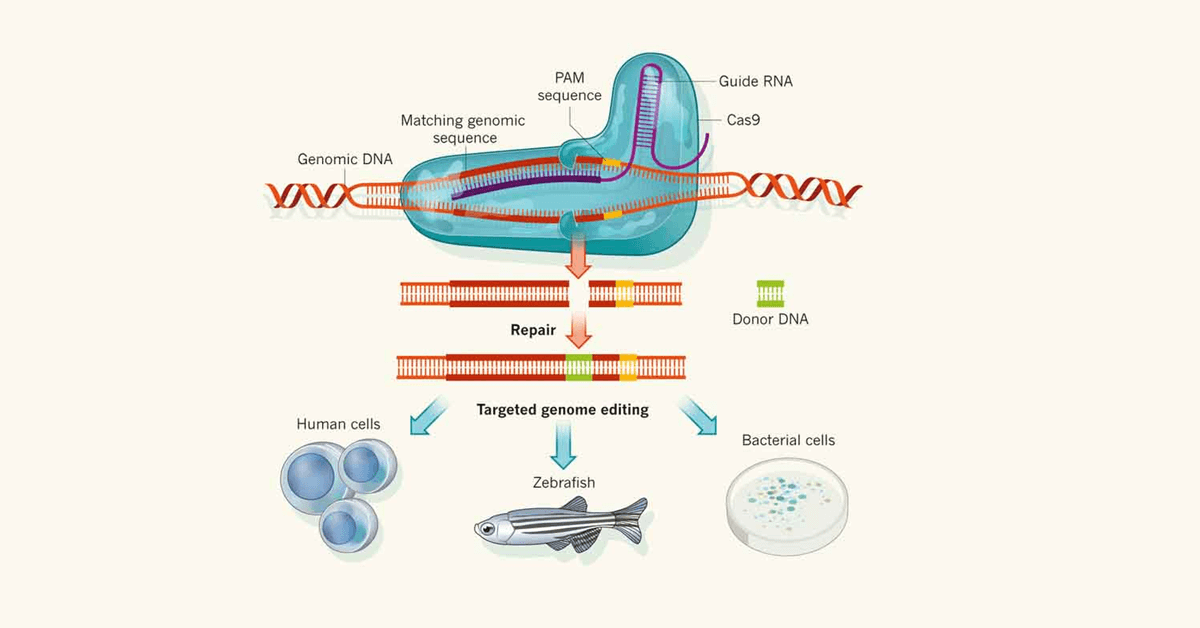
A publication was released summarizing one lab’s discovery that could lead to a future treatment for Fragile X.
Allos Pharma announced they held a meeting with the FDA to optimize the design of their Phase 3 trial designed to support an NDA to address FXS.
Research is needed that includes more racially and ethnically diverse participants. This study relied on self-reports to measure cognition.
From our NFXF Webinar Series, Dr. Berry-Kravis joined us and shared a brief presentation about what we’ve learned about the problems that face adults living with Fragile X syndrome from the FORWARD data.
RECONNECT is designed to confirm results of the first randomized, double-blind, placebo-controlled trial of ZYN002 conducted in FXS, CONNECT-FX.
We are sharing information on a 2023 funding opportunity from CDMRP and PRMRP.
In the first webinar in the NFXF’s 2023 Webinar Series, Dr. Tracy King and representatives from each of the current NIH-funded Fragile X Centers of Excellence shared updates and answered questions about their progress to date.
Healx shares the closing of their IMPACT-FXS trial with plans to open a new study in early 2023.
The main aim of this study was to determine whether boys with FXS who had participated in our previous 12-week randomized trial of telehealth-enabled behavioral treatments for challenging behaviors were still benefiting from having received the treatment several years later.
Chad Coberly, CEO of Tetra Therapeutics, a Shionogi Group Company, presented about BPN14770, a PDE4 inhibitor, during the Industry Updates keynote session at the 18th International Fragile X Conference.
This proposal is submitted on behalf of the NFXF Clinical Trials Committee. The committee will present examples of recent announcements and headlines focused on treatment development in Fragile X syndrome.
This presentation covers results from completed clinical trials and development programs for new medications in fragile X syndrome.
It’s that time of year! Our 2022 Industry Updates session, moderated by Hilary Rosselot, includes presentations from Asuragen, Zynerba Pharmaceuticals, Tetra Therapeutics, and Healx.
The Fragile X research landscape is always evolving. Understanding how the past has influenced the present is important, but what about our future?
FMR1 now stands for fragile X messenger ribonucleoprotein 1, removing the reference to “mental retardation” which has long been outdated in common vernacular. At the time of discovery, “mental retardation” was an accepted term for what we now call “intellectual disability.”
More research needs to be done, but knowing more about how FXS differs in people with and without methylation mosaicism may eventually help guide the expectations and treatment of individuals with FXS.
Findings showed that sleep difficulties are prevalent in children with FXS and, although they tend to be mild, they are associated with behavioral problems and negative impact to families.
On September 14, 2021, we held an NFXF webinar with three of our industry partners working on treatments for Fragile X syndrome. Each shared the most up-to-date information on their research projects in a way more easily understood by laypeople. A short Q&A followed each presentation.
The cooperative agreement was awarded to Dr Elizabeth Berry-Kravis of Rush University Medical Center by the Centers for Disease Control and Prevention.
Dr. Liz Berry-Kravis and Tetra Therapeutics published the BPN14770 trial results in Nature Medicine on April 29, 2021. We present a summary of those research results.
Patient-focused drug development meetings allow the FDA to hear directly from patients, their families, caregivers, and patient advocates. The NFXF PFDD meeting was held on March 3, 2021.
Dr. Berry-Kravis discusses what we’ve learned about the problems that face adults living with Fragile X syndrome.
Allos Pharma, a late-stage pharmaceutical company developing therapeutics for neurodevelopmental disorders, has announced the exclusive license rights to arbaclofen in Fragile X syndrome.
The NIH is the biggest funder of Fragile X research — around $40M per year. That is why their strategic plan to guide Fragile X research is so important.
Diane and her son Joshua volunteered for a clinical trial a little over a year ago. In this heartwarming video, his mom shares how their family made the decision to participate, and what the experience has been like so far.
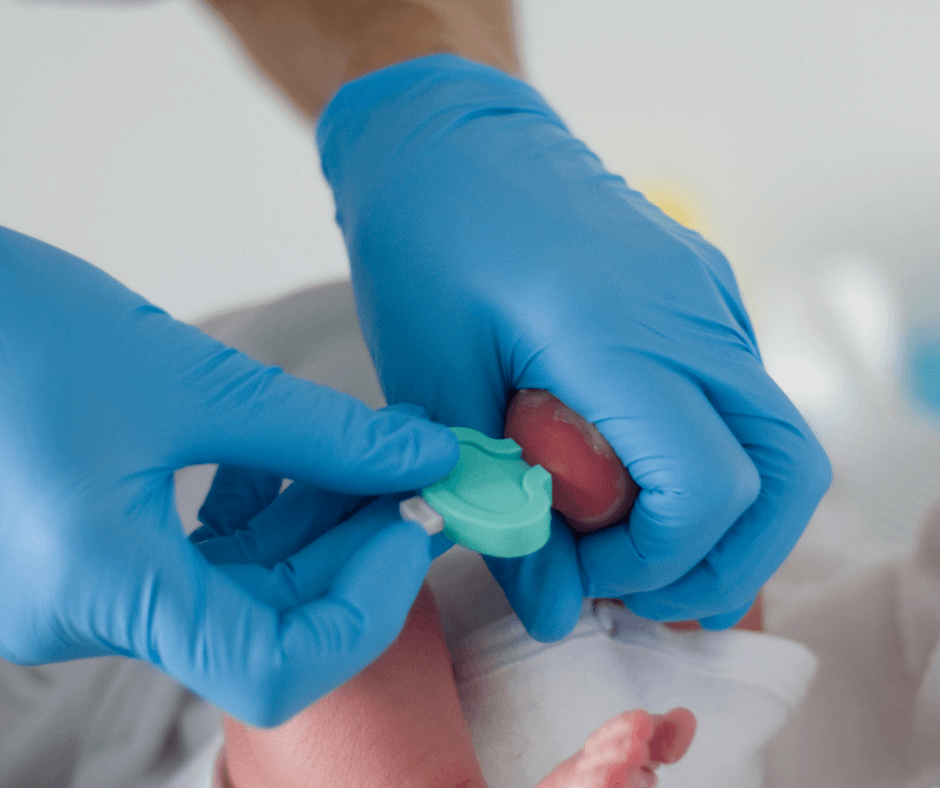
Early Check screening for Fragile X will give researchers important information about how Fragile X affects infants and toddlers before any signs or symptoms are noticed by parents or doctors. It provides very early developmental assessments and intervention. It will also help prove whether early diagnosis and intervention have long-term benefits for children with Fragile X syndrome.
Hilary Rosselot, NFXF executive director and research facilitation lead, gives advice and guidance to participating in research studies and clinical trials.
Our partners at the CDC released a new paper intending to help clinicians identify preventive care services that their patients with FXS may need.
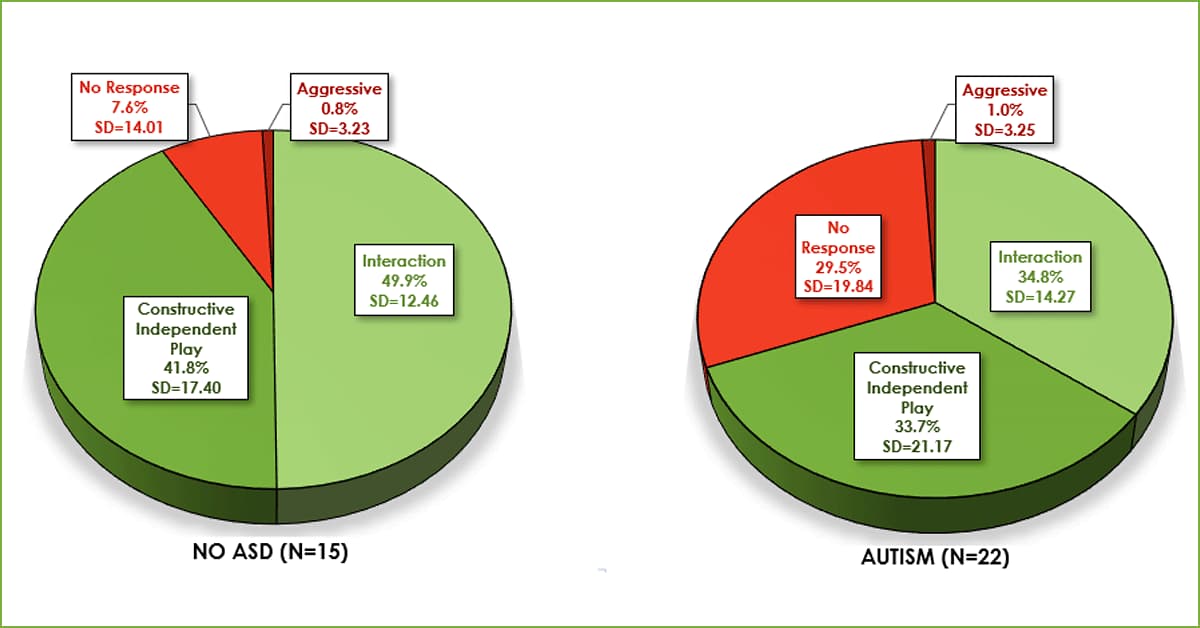
We take a look at behavioral issues in individuals with FXS plus ASD and compare them to those with FXS alone.
These findings provide preliminary information about the early language learning environment and support for the feasibility of utilizing an automated vocal analysis system within the FXS population that could ease data collection and further our understanding of the emergence of language development.
Early Check, a new research study, is now available for newborn babies in North Carolina. Fragile X syndrome is included in the screening.
Family caregivers affected by FXS are significantly more likely to report financial burden and quit their job or cut working hours than those with ASD or ID only.
The gene and protein responsible for causing Fragile X syndrome emerge as a leading candidate in the search for the cause of autism and maybe even schizophrenia.
Understanding screening issues and challenges is crucial to advancing the Fragile X field as it relates to diagnosis and treatment.