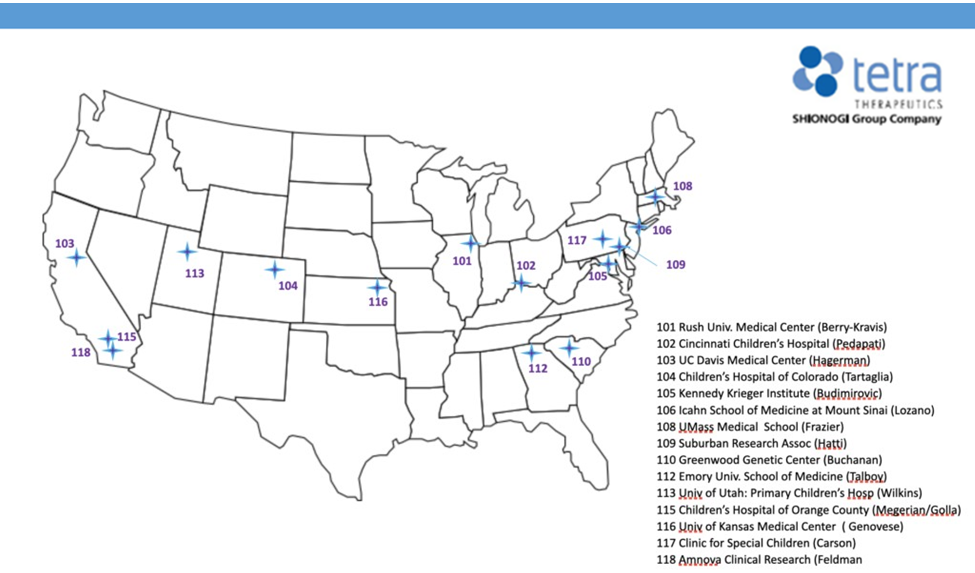
About the Study
Who can participate?
Males ages 9–45 may be eligible to participate with a molecular genetic confirmation of FXS: FMR1 >200 CGG repetitions.
What will happen in the study?
If the individual qualifies and decides to be in this research study, they will conduct eight visits (at clinic and at home) over 13 weeks.
The following is a list of some of the study procedures that will happen during the study:
- Participant cognitive and behavior testing
- Blood draws
- Parent/caregiver questionnaires
For more information, visit Tetra’s website.
What are the good things that can happen from this research?
Potential benefits are improvement in cognitive (language and vocabulary) function and improvement in daily activities/behavior.
What are the bad things that can happen from this research?
Potential drug side effects include mild nausea and diarrhea.
There may be other risks that we do not know about yet.
Will I or my child be paid to complete this study?
Participants receive $50 per visit (6).
Travel can be coordinated and paid for by Tetra or reimbursed for eligible families, even if there is not a site nearby.
Who can I contact for more information about this study?
There are several sites across the U.S. Complete the contact form below to notify the site nearest you!