A Message from Harmony Biosciences about the RECONNECT Trial and the conclusion of its Open Label Extension (OLE) program
The NFXF hosted an informative webinar featuring Dr. Elizabeth Berry-Kravis, who shared updates on the CDC-funded FORWARD-MARCH study and new insights into Fragile X syndrome and premutation health. The study builds on more than a decade of CDC-supported FORWARD Fragile X research, which began in 2012 and continues to collect nationwide data to advance understanding of Fragile X syndrome.
Dr. David Hessl joined the NFXF to discuss the exciting new ACT for FXTAS study, funded by the National Institute of Neurological Disorders and Stroke (NINDS).
Harmony Biosciences recently announced results from its Phase 3 RECONNECT study of ZYN002, a cannabidiol (CBD) gel being tested for Fragile X syndrome.
We’re on a mission to enroll 600 participants with FXS born in 2003 through 2020. We’re halfway there and we need your help!
In our first webinar in the NFXF’s 2025 Webinar Series, we hear updates from each of the current NIH-funded Fragile X Centers of Excellence. Why is this year’s Advocacy Day especially important? Congress is gearing up to make crucial decisions about our nation’s priorities and how to fund them. The NIH is one of those federal funding bodies supporting Fragile X research for over two decades.
Dr. Elizabeth Berry-Kravis and Dr. Walter Kaufmann presented two of the “most-cited” publications from the American Journal of Medical Genetics Part A and answered questions during a moderated Q&A.
Harmony Biosciences shares that they have reached their enrollment goal for the Phase 3 RECONNECT trial and screening into the trial has officially closed.
A message about Shionogi’s EXPERIENCE clinical trials from Dr. Juan-Carlos Gomez, Chief Medical Officer at Shionogi & Co., Ltd.
You Spoke, We Listened: The Completely At Home RECONNECT Clinical Trial –Participate in Research Without the Stress of Traveling
You may have heard about EXPERIENCE (Evaluation of Fragile X Experience in Cognition Expression) clinical trials as the Tetra studies or the studies of BPN14770 in Fragile X syndrome. EXPERIENCE is now being managed by Shionogi and clinical trial sites across the U.S. are still enrolling qualified male participants aged 9-45.
We live in a day and age of social sharing, and that is not going anywhere. However, when we participate in a clinical trial, we have to understand that we cannot share everything in order to protect the integrity of the trial.
In the latest from the NFXF Webinar Series, panelists discuss the advances made in understanding Fragile X Syndrome through FORWARD, the Fragile X Online Registry With Accessible Research Database.
Kaerus Bioscience has completed its Phase 1 trial with its novel BK channel modulator, KER-0193, being developed for Fragile X Syndrome!
This report, from Summer Scholar Marwa Zafarullah, is based on a study to help us better understand the impact of the CGG repeats on the different clinical premutation phenotypes and the molecular mechanisms behind the development of Fragile X-associated disorders.
During this 45-minute Lunch & Learn webinar, Dr. Erickson discussed details of a single-dose medication study in Fragile X syndrome (FXS), including how the study was done, what the results showed, and moreover, why the results are important, what they mean for the FXS community, and next steps to move us forward.
In the second webinar in the NFXF’s 2024 Webinar Series, Dr. Tracy King and representatives from each of the current NIH-funded Fragile X Centers of Excellence shared updates and answered questions about their progress to date.
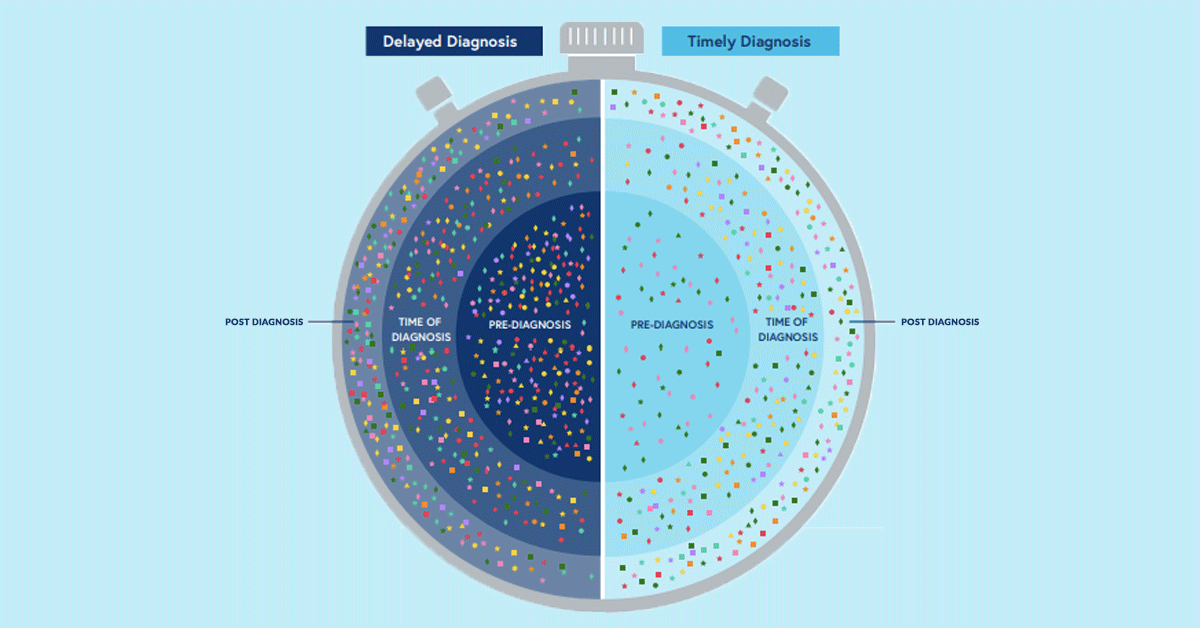
We are proud to be part of the Everylife Foundation’s newest report, which uses real-world data to evaluate healthcare usage in seven rare diseases and its relationship to timely diagnosis and the diagnostic odyssey.
In this three-minute video, Dr. Liz Berry-Kravis shares what participants are asked to do during the trial, who is eligible, and how to learn more.
More research is needed to better understand the progression of FMRP expression across the lifespan and how FMRP is related to all aspects of functioning.
Allos Pharma announced they held a meeting with the FDA to optimize the design of their Phase 3 trial designed to support an NDA to address FXS.
From our NFXF Webinar Series, Dr. Berry-Kravis joined us and shared a brief presentation about what we’ve learned about the problems that face adults living with Fragile X syndrome from the FORWARD data.
RECONNECT is designed to confirm results of the first randomized, double-blind, placebo-controlled trial of ZYN002 conducted in FXS, CONNECT-FX.
In the first webinar in the NFXF’s 2023 Webinar Series, Dr. Tracy King and representatives from each of the current NIH-funded Fragile X Centers of Excellence shared updates and answered questions about their progress to date.
Healx shares the closing of their IMPACT-FXS trial with plans to open a new study in early 2023.
Chad Coberly, CEO of Tetra Therapeutics, a Shionogi Group Company, presented about BPN14770, a PDE4 inhibitor, during the Industry Updates keynote session at the 18th International Fragile X Conference.
This proposal is submitted on behalf of the NFXF Clinical Trials Committee. The committee will present examples of recent announcements and headlines focused on treatment development in Fragile X syndrome.
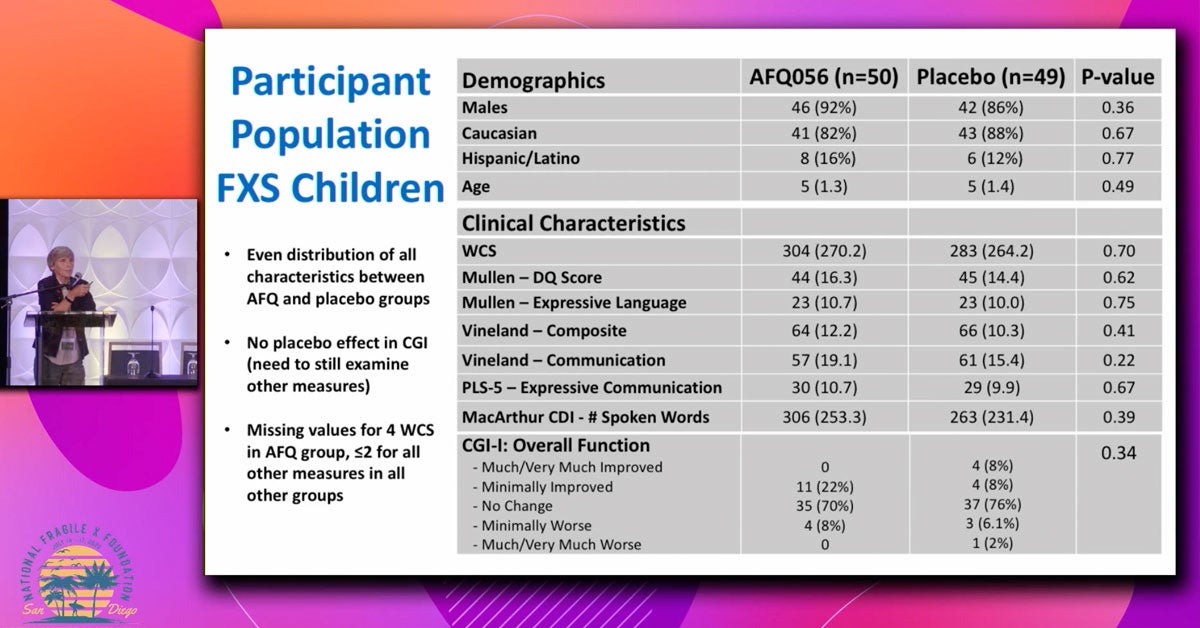
This presentation covers results from completed clinical trials and development programs for new medications in fragile X syndrome.
The Fragile X research landscape is always evolving. Understanding how the past has influenced the present is important, but what about our future?
Diane and her son Joshua volunteered for a clinical trial a little over a year ago. In this heartwarming video, his mom shares how their family made the decision to participate, and what the experience has been like so far.
Hilary Rosselot, NFXF executive director and research facilitation lead, gives advice and guidance to participating in research studies and clinical trials.
Early Check, a new research study, is now available for newborn babies in North Carolina. Fragile X syndrome is included in the screening.