Research News & Results
We are excited to share with you some of the great published research results. We are committed to keeping the community up-to-date on research findings, taking time to reflect and celebrate successes in the field of Fragile X-associated conditions and disorders.
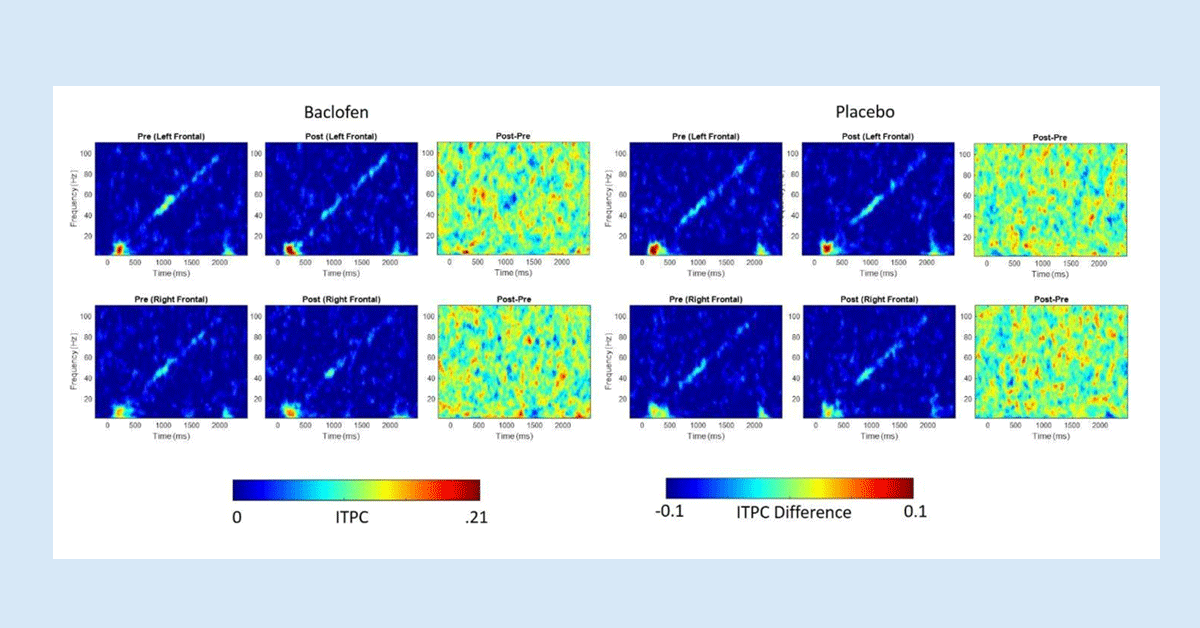
Research Results
A Note for Researchers: If you’ve recently published your scientific findings in a peer-reviewed journal, please share your work with us by emailing research@fragilex.org. Cheers to the amazing scientific advancements in our field and the advancements to come. It would not be possible without your dedication, passion, and drive to make each day just a bit better.
Research News
↓ Stay up-to-date by subscribing to our newsletter