Consensus of the Fragile X Clinical & Research Consortium
Understanding Autism Spectrum Disorder in Fragile X Syndrome
Reading Time: 59 min.—|—Last Updated: Dec 2020—|—First Published: Nov 2014—|—Download PDF
Families, and in some cases professionals, are often not clear about the relationship between fragile X syndrome (FXS) and autism spectrum disorder (ASD). It is not uncommon for a child to initially be diagnosed with FXS and later to receive an additional diagnosis of ASD or vice versa.
This document attempts to bring clarity to how the ASD diagnosis and FXS can overlap, and where they do not. Understanding this distinction can be particularly helpful for genetic counseling and when deciding upon the most appropriate medical, therapeutic, educational, and behavioral interventions that will increase the potential for both short-term and long-term improvements. At the end of this document, readers will find general treatment recommendations based on the current expert consensus and evidence-based publications in the field of ASD in FXS.
What is Autism Spectrum Disorder?
ASD is a neurodevelopmental disorder characterized by social communication challenges and the presence of restricted and repetitive behaviors. ASD symptoms usually appear in early childhood and change over time and with development.
The diagnosis of ASD is based on observations and assessments of behavior by an experienced clinician, where clinical judgment is informed by the use of standardized diagnostic instruments (Lord et al., 2006; Risi et al., 2006) and where there is a focus on core social communication and interaction and restricted and repetitive behavior symptoms. The gold standard for clinical diagnosis of ASD in FXS involves the use of the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5; American Psychiatric Association, 2013) or the International Classification of Diseases, 11th Edition (ICD-11).
In earlier DSM-based guidelines (DSM-IV), ASD was represented by three different diagnoses: autistic disorder; Asperger syndrome; and pervasive developmental disorder, not otherwise specified (PDD-NOS). These three diagnoses were combined into the one diagnosis of ASD under the DSM-5. The term, “spectrum,” in ASD means that people can be affected in different ways, and symptoms can range from having mild to severe impacts on a person’s functioning.
The symptoms of ASD do not result solely from limited communication skills or intellectual disability (ID), though both may be present. Instead, ASD represents a difficulty in using communication and intellectual abilities for social interaction. ASD involves a number of behaviors, as described in the bullet points below.
Social communication and social interaction symptoms in ASD include challenges with:
- Social-emotional reciprocity (e.g., having a conversation, sharing interests and emotions).
- Nonverbal communication used for social interaction (e.g., differences in use of eye contact, facial expressions and/or gestures).
- Developing and understanding social relationships (e.g., friendships, understanding rules for social behavior).
Restricted, repetitive behaviors include:
- Stereotyped or repetitive motor movements (e.g., hand flapping), use of objects (e.g., spinning or lining up toys) and/or speech (using repetitive and/or unusual words or phrases).
- Insistence on sameness and/or ritualized patterns of behavior (e.g., extreme distress at small changes, difficulties with transitions, needing to follow the same schedule or sequence of completing activities).
- Unusual or overly intense interests (e.g., interest in an unusual object or topic, intense interest in a narrow area).
- Hyper- or hypo-reactivity to sensory input (e.g., negative reactions to certain textures or sounds; excessive smelling, peering at, or touching of objects).
When diagnosing ASD, the clinician should state whether it is associated with a known medical, genetic, or environmental factor. They should also specify whether ASD is with or without accompanying ID and with or without accompanying language delays.
What is Known About the Cause of ASD?
ASD is a complex condition with a common set of behavioral symptoms, but with varied, yet not well understood, underlying risk factors and biological mechanisms. Although people with ASD share core features of social interaction challenges and restricted, repetitive behaviors, these symptoms can vary widely in form and severity. Further complicating the picture, many individuals with ASD have co-occurring conditions, such as language disorders, ID, or mental health diagnoses (e.g., ADHD, anxiety). Among the Causes and Risk Factors noted by both the U.S. Centers for Disease Control (CDC) and the National Institutes of Health (NIH):
- Research has identified around 100 high-confidence risk genes (Iossifov et al., 2014; Yuen et al., 2017), but many are yet to be discovered (Feliciano et al., 2019).
- Children who have a sibling with ASD are at a higher risk of also having ASD (Ozonoff et al., 2011).
- Individuals with certain genetic or chromosomal conditions, such as FXS, can have a greater chance of having ASD.
It is expected that as genetic testing becomes more sensitive, the percentage of individuals with an identified genetic cause of ASD will increase further (Feliciano et al., 2019). However, at this time, there is no medical test, such as a blood test or brain scan that can diagnose ASD.
What is Known About the Relationship Between FXS and ASD?
FXS is the most common single gene disorder associated with ASD, accounting for about 1-6% of all cases of ASD [Muhle et al., 2004; Schaefer & Mendelsohn, 2008]. FXS is caused by an expansion of a CGG repeat sequence to >200 repeats (full mutation) in the promoter region of the FMR1 gene, which results in a loss of its encoded protein, the Fragile X Messenger Ribonucleoprotein (FMRP) [Hagerman et al., 2009].
FXS is diagnosed by a DNA blood test, unlike ASD, which is a purely behaviorally defined diagnosis. The behavioral characteristics of FXS, although quite variable, can include many features of ASD, such as difficulties with social interaction and communication (e.g., poor eye contact, problems with peer relationships, social withdrawal) (Budimirovic & Kaufmann, 2011), repetitive movements (e.g., hand flapping), need for sameness, and self-injurious behavior (e.g., hand biting) (Hagerman, 2002). Thus, careful discernment and a clinical evaluation are needed to understand whether ASD is present in an individual with FXS.
Many individuals diagnosed with FXS meet criteria for a diagnosis of ASD, with the estimated prevalence of ASD in FXS being approximately 50% for males and 20% for females (Bailey et al. 2008; Clifford et al., 2007; Kaufmann et al., 2017). However, recent research (Wheeler et al., 2015) indicates that, while a majority of males and females with FXS have restricted and repetitive behaviors common to ASD, far fewer meet criteria for the social communication and interaction problems associated with ASD. Receiving a diagnosis of FXS and ASD can be appropriate, but the relationship between FXS and ASD and the characteristics of ASD seen in FXS are still an evolving science and the diagnosis of ASD in FXS continues to be studied by clinicians and researchers working with people with FXS.
Many individuals diagnosed with FXS meet criteria for a diagnosis of ASD, with the estimated prevalence of ASD in FXS being approximately 50% for males and 20% for females.
Why Are Autistic Features Found in FXS?
Our current knowledge about ASD indicates that it is a developmental brain disorder, beginning shortly after birth or even earlier. Its most characteristic feature is the presence of abnormal patterns of neural “wiring” or connectivity. Because multiple genetic and environmental factors have been linked to ASD [Fernandez and Scherer, 2017], there are probably multiple ways in which neural connectivity and other processes can be disrupted, leading to ASD (Lord et al., 2020).
There is evidence, based on studies of genes and proteins in neural cells, that FMRP plays a role in the processes that lead to ASD. FMRP, which is absent or deficient in FXS, normally turns off protein synthesis (the process of proteins being made) at the level of connections between neurons (dendrites). When certain receptors (such as mGluR, dopamine D2, and muscarinic cholinergic) are activated, this releases the FMRP brake and allows proteins to be made. In this way, FMRP regulates the levels of many proteins important at brain connections. Also, many proteins that have similar functions to FMRP, and also interact with FMRP, have been found to be associated with ASD [De Rubeis & Bagni, 2011; Darnell et al., 2011]. In addition, many of the proteins regulated by FMRP have been found to be associated with ASD [Iossifov et al., 2012; Ascano et al., 2012]. Thus, deficits in FMRP seem to be linked to abnormal wiring and related brain abnormalities that lead to behavioral symptoms of ASD.
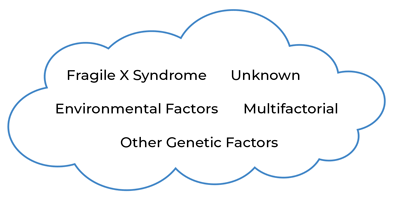
Figure 1: Multiple causes and contributors result in ASD.
It may be helpful to think of ASD as a “cloud” (see Figure 1), where multiple diverse genetic and other causes lead to atypical brain wiring that results in a final common diagnostic category. Some causes may stem from a single genetic change, some causes may require a number of factors to interact together (i.e., multifactorial), and some causes may be unknown. Although the causes can vary, the end result is the set of behaviors known as ASD.
Individuals with FXS who meet criteria for the current definition of ASD represent an area in the ASD “cloud” where an individual may meet for ASD criteria due to reduced social interactions with others, reduced interest in social interaction, and increased repetitive behaviors in addition to the common characteristics associated with FXS, such as social anxiety, intellectual disability, and sensory hyperreactivity (Hall et al., 2010). However, not all individuals with FXS meet criteria for the diagnosis of ASD. FXS is not a kind of ASD; rather, it is a genetic disorder with its own set of symptoms, many of which overlap with behavioral symptoms seen in ASD. When enough overlapping symptoms are present and severe enough to impact functioning, the person with FXS could also meet criteria for a diagnosis of ASD. Some individuals with FXS may have enough ASD features to be near the ASD cloud, but not at the threshold needed for an ASD diagnosis, and those with FXS who are in the ASD cloud likely have genetic factors besides FMRP deficiency that contribute to the development of ASD.
Intellectual disability is an important variable in considering whether ASD is present within FXS. ID, a feature of FXS in most males, is common in ASD but is not necessary for a diagnosis of ASD, nor for FXS with ASD. However, severe ID is often difficult to differentiate from ASD, as it, too, includes challenges in social development and communication, and repetitive and self-stimulatory behaviors are often present.
The DSM-5 requires consideration of overall intellectual level in determining an ASD diagnosis. If a child’s social communication delays are in line with their overall developmental level, then a diagnosis of ASD is not warranted. In other words, social challenges must exceed those expected based on a person’s overall developmental level.
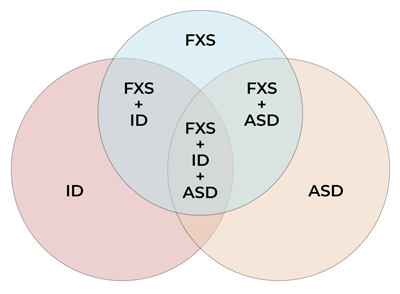
Figure 2: Venn diagram displaying set relationships between FXS, ID, and ASD (size of overlaps only approximate).
Figure 2 is a Venn diagram used to illustrate the relationship between ASD and FXS but it is not designed to be proportional.
Similarities and Differences
Neurological and behavioral characteristics common to both FXS and ASD include:
- Social interaction problems, including being able to accurately read and respond to social situations.
- Communication/language delays.
- in FXS involving a wide range of language and speech aspects
- in ASD mainly involving non-verbal communication and language pragmatics (use of language in social situations).
- Poor eye contact (though this often improves in FXS as the person gets to the know the other person).
- Repetitive use of objects and repetitive movements.
- Intellectual disability in most males and some females with FXS and a high proportion of those with ASD.
- Unusual reactions to sensory input, including hyper-sensitivity (e.g., tactile defensiveness) and hypo-sensitivity (e.g., high tolerance for pain, seeking sensory input such as visual fascination with moving objects or lights), the latter more common in ASD.
- An area of strength in visual memory.
- Seizures in a higher proportion than the general population.
- Motor coordination difficulties (e.g., challenges with handwriting, atypical walking patterns).
- Difficulties with attention, activity level, emotional-behavioral regulation and mood, often leading to additional diagnoses.
- Other behavioral issues (e.g., aggression, noncompliance, self-injury).
- Sleep problems.
While both ASD and FXS (without ASD) involve social challenges, clinicians working with individuals with FXS observe distinct differences in that lack of social initiations alone do not necessarily imply the absence of social awareness or social interest in individuals with FXS.
For most individuals with FXS, lack of social initiation often reflects language delays, intellectual disabilities, and general or social anxiety.
In contrast, lack of social initiation in ASD, including individuals with FXS who meet criteria for ASD, are defined based on reduced interest in social interaction or a failure to attend to social information that might promote social behavior.
A similar trend is found for eye contact. Although poor eye contact is characteristic of both FXS and ASD, the nature of eye contact may be substantially different.
Individuals with FXS typically avoid eye contact directly (Watson et al., 2008; Hall et al, 2009), and looking away from people may help in coping with emotional discomfort that is driven by underlying social anxiety (Budimirovic & Kaufmann, 2011).
While social anxiety might also play a role in some individuals with ASD, including those with FXS who meet criteria for ASD, other individuals with ASD make little eye contact for other reasons, such as a lack of recognition that eye gaze is a source of social information or interaction [Hoeft et al., 2011; Hazlett et al., 2012].
Characteristics that have been found to differ between FXS and ASD include:
- Individuals with ASD typically have higher levels of motor coordination.
- Individuals with ASD are more likely to show higher expressive language skills relative to receptive language (Ellis Weismer et al., 2010), while individuals with FXS tend to show higher receptive language skills relative to expressive (Abbeduto et al., 2007).
- In general, interest in socializing is higher in FXS than ASD, though anxiety and language delays can be limiting factors (Niu et al., 2017; Ellis et al., 2020).
- Social awareness is typically better in FXS than ASD.
- Motor imitation skills are typically better in FXS than ASD (Macedoni-Luksic et al., 2009; Marschik et al., 2014).
- Sequential processing is typically harder for individuals with FXS than ASD. (Hodapp et al. 1992, 1993)
Characteristics of Individuals with FXS with ASD
Outlined above were descriptions of behaviors that are common and different between FXS and ASD. Compared to individuals with FXS who do not meet criteria for ASD, those who do meet ASD criteria present with more severe cognitive and behavioral problems that include:
- Less developed language skills, particularly receptive skills (Abbeduto et al., 2007; McDuffie et al., 2012).
- Often non-verbal or minimally verbal (McDuffie et al., 2012).
- Lower IQ scores (Niu et al., 2017; Kaufmann et al., 2004; Budimirovic et al., 2006, 2020).
- Lower adaptive skills (Kaufmann et al., 2004; Budimirovic et al., 2006).
- More severe overall behavioral problems: attention problems, hyperactivity and impulsivity, anxiety (particularly after childhood), irritability/aggression/agitation/self-injury, hypersensitivity to stimuli, and obsessive compulsive/perseverative behavior (Budimirovic et al., 2006; Eckert et al., 2019; Kaufmann et al., 2017).
- Sleep problems, particularly after childhood (Kaufmann et al., 2017).
- Seizures (Berry-Kravis et al., 2017; Kaufmann et al., 2017).
It is interesting to note that the above list contains characteristics that are not core features of ASD. Rather, they reflect common comorbidities and/or greater developmental impairment. It is important to note that many of these characteristics are seen in individuals with FXS with significant ID, who do not have ASD. Therefore, the diagnosis of ASD can be difficult to make in these individuals with FXS and significant ID. So, when making the ASD diagnosis in FXS, consideration must be given to the cognitive level of the individual to ensure that ASD symptoms (social communication challenges and repetitive behaviors) are more impaired than expected based on overall developmental level.
Future studies are needed to improve methods for diagnosis of ASD in those with ID (Kidd et al., 2020). Using data from the FORWARD project (A CDC-funded, multi-year, natural history study involving multiple Fragile X clinics), revised questionnaires for the diagnosis of ASD that are more appropriate for FXS have been developed (Kidd et al., 2020), and further work is needed to refine ASD diagnostic measures for use in individuals with FXS.
From the educational, vocational, and adaptive functioning viewpoints, individuals with FXS with ASD face similar but more severe challenges than those with FXS without ASD. Individuals with FXS with ASD have a higher frequency of use of medications for behavioral management (Kaufmann et al., 2017, Erickson et al., 2019), as well as higher likelihood of having very delayed toilet training, seizures, and sleep problems (Berry-Kravis et al., 2019; Kaufmann et al., 2017).
Failure to accurately identify ASD in FXS has multiple implications including that developmental and neurobehavioral comorbidities associated with ASD in FXS may not be recognized, leading to failure to enact preventive strategies. An inadequate diagnosis may also prevent the implementation of interventions and educational strategies shown to have benefit in ASD, such as applied behavior analysis (ABA). More precise delineation of ASD behaviors exhibited by an individual with FXS could also assist in developing targeted interventions (Kaufmann et al., 2017). Therefore, accurate and early diagnosis and prompt referral of children with FXS and ASD to receive appropriate therapies and supports is essential to make an early and positive impact on outcomes.
Closing
Up to half of the people with FXS will also have a behavioral diagnosis of ASD, and it can be useful to get the dual diagnosis early as it may impact treatment and intervention. It is important to note that, while many people with FXS with ASD will have increased challenges, they may also have the positive attributes of those with FXS without ASD including having a good sense of humor, a desire to engage with others, and to be helpful. It is important to use those strengths to further their academic and life skills. Regardless of ASD status, evaluations and treatment plans work best when they are individualized to an individual’s specific needs and profile of strengths and weaknesses.
Questions?
If you’re a parent or caregiver and have questions about the information presented here, we’d love to hear from you! You can reach out to Missy Zolecki using the contact info or our contact form below.

Missy Zolecki,
Community Empowerment
treatment@fragilex.org
(800) 688-8765
references
Abbeduto, L., Brady, N., & Kover, S. T. (2007). Language development and fragile X syndrome: profiles, syndrome-specificity, and within-syndrome differences. Mental retardation and developmental disabilities research reviews, 13(1), 36–46.
American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders, 5th Edition. Arlington, VA: American Psychiatric Publishing.
Ascano, M., Jr, Mukherjee, N., Bandaru, P., Miller, J. B., Nusbaum, J. D., Corcoran, D. L., Langlois, C., Munschauer, M., Dewell, S., Hafner, M., Williams, Z., Ohler, U., & Tuschl, T. (2012). FMRP targets distinct mRNA sequence elements to regulate protein expression. Nature, 492(7429), 382–386.
Bagni, C., & Greenough, W. T. (2005). From mRNP trafficking to spine dysmorphogenesis: the roots of fragile X syndrome. Nature reviews. Neuroscience, 6(5), 376–387.
Baron-Cohen, S. (2013, May 30). Despite fears, DSM-5 is a step forward. [Web log post.] Retrieved from http://sfari.org/news-and-opinion/specials/2013/dsm-5-special-report/despite-fears-dsm-5-is-a-step-forward
Barton, M. L., Robins, D. L., Jashar, D., Brennan, L., & Fein, D. (2013). Sensitivity and specificity of proposed DSM-5 criteria for autism spectrum disorder in toddlers. Journal of autism and developmental disorders, 43(5), 1184–1195.
Berry-Kravis, E., Kidd, S. A., Lachiewicz, A. M., Choo, T. H., Tartaglia, N., Talapatra, D., Aguirre-Kolb, C., Andrews, H., & Riley, K. (2019). Toilet Training in Fragile X Syndrome. Journal of developmental and behavioral pediatrics : JDBP, 40(9), 751–761.
Berry-Kravis, E., Raspa, M., Loggin-Hester, L., Bishop, E., Holiday, D., & Bailey, D. B. (2010). Seizures in fragile X syndrome: characteristics and comorbid diagnoses. American journal on intellectual and developmental disabilities, 115(6), 461–472.
Budimirovic, D. B., Bukelis, I., Cox, C., Gray, R. M., Tierney, E., & Kaufmann, W. E. (2006). Autism spectrum disorder in Fragile X syndrome: differential contribution of adaptive socialization and social withdrawal. American journal of medical genetics. Part A, 140A(17), 1814–1826.
Budimirovic, D. B., & Kaufmann, W. E. (2011). What can we learn about autism from studying fragile X syndrome?. Developmental neuroscience, 33(5), 379–394.
Budimirovic, D.B.; Schlageter, A.; Filipovic-Sadic, S.; Protic, D.D.; Bram, E.; Mahone, E.M.; Nicholson, K.; Culp, K.; Javanmardi, K.; Kemppainen, J.; Hadd, A.; Sharp, K.; Adayev, T.; LaFauci, G.; Dobkin, C.; Zhou, L.; Brown, W.T.; Berry-Kravis, E.; Kaufmann, W.E.; Latham, G.J. A Genotype-Phenotype Study of High-Resolution FMR1 Nucleic Acid and Protein Analyses in Fragile X Patients with Neurobehavioral Assessments. Brain Sciences. 2020, 10, 694.
Carey, B. (2012, January 19). New definitions of autism will exclude many, study suggests. The New York Times. Retrieved from http://www.nytimes.com
Centers for Disease Control and Prevention. (2012). Prevalence of autism spectrum disorders—Autism and Developmental Disabilities Monitoring Network, United States, 2008. Morbidity and Mortality Weekly Report (Report No.61[SS03)]). Washington, DC: Government Printing Office.
Darnell, J. C., Van Driesche, S. J., Zhang, C., Hung, K. Y., Mele, A., Fraser, C. E., Stone, E. F., Chen, C., Fak, J. J., Chi, S. W., Licatalosi, D. D., Richter, J. D., & Darnell, R. B. (2011). FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell, 146(2), 247–261.
De Rubeis, S., & Bagni, C. (2011). Regulation of molecular pathways in the Fragile X Syndrome: insights into Autism Spectrum Disorders. Journal of neurodevelopmental disorders, 3(3), 257–269.
Eckert, E. M., Dominick, K. C., Pedapati, E. V., Wink, L. K., Shaffer, R. C., Andrews, H., Choo, T. H., Chen, C., Kaufmann, W. E., Tartaglia, N., Berry-Kravis, E. M., & Erickson, C. A. (2019). Pharmacologic Interventions for Irritability, Aggression, Agitation and Self-Injurious Behavior in Fragile X Syndrome: An Initial Cross-Sectional Analysis. Journal of autism and developmental disorders, 49(11), 4595–4602.
Ellis, K., Oliver, C., Stefanidou, C., Apperly, I., & Moss, J. (2020). An Observational Study of Social Interaction Skills and Behaviors in Cornelia de Lange, Fragile X and Rubinstein-Taybi Syndromes. Journal of autism and developmental disorders, 50(11), 4001–4010.
Feliciano, P., Zhou, X., Astrovskaya, I., Turner, T. N., Wang, T., Brueggeman, L., Barnard, R., Hsieh, A., Snyder, L. G., Muzny, D. M., Sabo, A., SPARK Consortium, Gibbs, R. A., Eichler, E. E., O’Roak, B. J., Michaelson, J. J., Volfovsky, N., Shen, Y., & Chung, W. K. (2019). Exome sequencing of 457 autism families recruited online provides evidence for autism risk genes. NPJ genomic medicine, 4, 19.
Fernandez, B. A., & Scherer, S. W. (2017). Syndromic autism spectrum disorders: moving from a clinically defined to a molecularly defined approach. Dialogues in clinical neuroscience, 19(4), 353–371.
Frazier, T. W., Youngstrom, E. A., Speer, L., Embacher, R., Law, P., Constantino, J., Findling, R. L., Hardan, A. Y., & Eng, C. (2012). Validation of proposed DSM-5 criteria for autism spectrum disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 51(1), 28–40.e3.
Frith C. (2004). Is autism a disconnection disorder?. The Lancet. Neurology, 3(10), 577.
Gannon, C. E., Britton, T. C., Wilkinson, E. H., & Hall, S. S. (2018). Improving social gaze behavior in fragile X syndrome using a behavioral skills training approach: a proof of concept study. Journal of neurodevelopmental disorders, 10(1), 25.
Gibbs, V., Aldridge, F., Chandler, F., Witzlsperger, E., & Smith, K. (2012). Brief report: an exploratory study comparing diagnostic outcomes for autism spectrum disorders under DSM-IV-TR with the proposed DSM-5 revision. Journal of autism and developmental disorders, 42(8), 1750–1756. doi: 0.1007/s10803-012-1560-6.
Grant, R., & Nozyce, M. (2013). Proposed changes to the American Psychiatric Association diagnostic criteria for autism spectrum disorder: implications for young children and their families. Maternal and child health journal, 17(4), 586–592.
Hagerman R.J.: The physical and behavioral phenotype; in Hagerman R.J., Hagerman P.J. (eds) (2002). Fragile X Syndrome: Diagnosis, Treatment, and Research, ed 4. Baltimore, Johns Hopkins University Press, pp 3–110.
Hagerman, R. J., Berry-Kravis, E., Kaufmann, W. E., Ono, M. Y., Tartaglia, N., Lachiewicz, A., Kronk, R., Delahunty, C., Hessl, D., Visootsak, J., Picker, J., Gane, L., & Tranfaglia, M. (2009). Advances in the treatment of fragile X syndrome. Pediatrics, 123(1), 378–390.
Hall, S.S., Lightbody, A. A., Hirt, M., Rezvani, A., & Reiss, A.L. (2010). Autism in Fragile X Syndrome: a category mistake? Journal of the American Academy of Child and Adolescent Psychiatry, 54, 921-933.
Hall, S.S., Monlux, K.D., Rodriguez, A. B., Jo, B., Pollard, J. S., (in press). Telehealth-Enabled Behavioral Treatment for Problem Behaviors in Boys with Fragile X Syndrome: A Randomized Controlled Trial. Journal of Neurodevelopmental Disorders.
Happé, F., & Frith, U. (1991). Debate and argument: How useful is the “PDD” label? Journal of Child Psychology and Psychiatry and Allied Disciplines 32(7), 1167-1168.
Hartley, S. L., & Sikora, D.M. (2009). Which DSM-IV-TR criteria best differentiate high-functioning autism spectrum disorder from ADHD and anxiety disorders in older children? Autism, 13(5), 485-509.
Hatton, D.D., Sideris, J., Skinner, M., Mankowski, J., Bailey, D.B. Jr (2006). Autistic behavior in children with fragile X syndrome: prevalence, stability, and the impact of FMRP. American Journal of Medical Genetics, 140A, 1804–1813.
Hazlett, H.C., Poe, M.D., Lightbody, A.A., Styner, M., MacFall, J.R., Reiss A.L., & Piven J. (2012). Trajectories of early brain volume development in fragile X syndrome and autism. Journal of the American Academy of Child & Adolescent Psychiatry, Sep;51(9):921-33. doi: 10.1016/j.jaac.2012.07.003. Epub 2012 Aug 1.
Hodapp, R. M., Leckman, J. F., Dykens, E. M., Sparrow, S. S., Zelinsky, D. G., & Ort, S. I. (1992). K-ABC profiles in children with fragile X syndrome, Down syndrome, and nonspecific mental retardation. American Journal on Mental Retardation, 97(1), 39–46.
Hodapp, R. M., Dykens, E. M., Ort, S. I., Zelinsky, D. G., & Leckman, J. F. (1991). Changing patterns of intellectual strengths and weaknesses in males with fragile X syndrome. Journal of Autism and Developmental Disorders, 21(4), 503-516.
Hoeft, F., Walter, E., Lightbody, A.A., Hazlett, H.C., Chang, C., Piven, J., Reiss, A., (2011). Neuroanatomical differences in toddler boys with fragile X syndrome and idiopathic autism. Archives Of General Psychiatry, 68, 295–305.
Howlin, P. (2003). Outcome in high-functioning adults with autism with and without early language delays: implications for the differentiation between autism and Asperger syndrome. Journal of Autism and Developmental Disorders, 33(1), 3-13.
Huerta, M., Bishop, S. L., Duncan, A., Hus, V., & Lord, C. (2012). Application of DSM-5 criteria for autism spectrum disorder to three samples of children with DSM-IV diagnoses of pervasive developmental disorders. The American Journal of Psychiatry, 169(10), 1056-1064. doi: 10.1176/appi.ajp.2012.12020276
Hus, V., Pickles, A., Cook Jr, E. H., Risi, S., & Lord, C. (2007). Using the autism diagnostic interview—revised to increase phenotypic homogeneity in genetic studies of autism. Biological psychiatry, 61(4), 438-448.
Hyman, S. L., Levy, S. E., & Myers, S. M. (2020). Identification, Evaluation, and Management of Children With Autism Spectrum Disorder. Pediatrics, 145(1), e20193447. doi: 10.1542/peds.2019-3447
Iossifov, I., Ronemus, M., Levy, D., Wang, Z., Hakker, I., Rosenbaum, J., Yamrom, B., Lee, Y.H., Narzisi, G., Leotta, A., Kendall, J., Grabowska, E., Ma, B., Marks, S., Rodgers, L., Stepansky, A., Troge, J., Andrews, P., Bekritsky, M., Pradhan, K., Ghiban, E., Kramer, M., Parla, J., Demeter, R., Fulton, L.L., Fulton, R.S., Magrini, V.J., Ye, K., Darnell, J.C., Darnell, R.B., Mardis, E.R., Wilson, R.K., Schatz, M.C., McCombie, & W.R., Wigler, M. (2012) De novo gene disruptions in children on the autistic spectrum. Neuron 74:285–299
Iossifov, I., O’roak, B. J., Sanders, S. J., Ronemus, M., Krumm, N., Levy, D., … & Smith, J. D. (2014). The contribution of de novo coding mutations to autism spectrum disorder. Nature, 515(7526), 216-221.
Kaufmann, W.E., Cortell, R., Kau, A.M., Bukelis, I., Tierne.y E., Gray, R.M., Cox, C., Capone, G.T., & Stanard, P. (2004). Autism spectrum disorder in fragile X syndrome: communication, social interaction, and specific behaviors. American Journal of Medical Genetics, 129A, 225–234.
Kaufmann, W.E., Capone, G., Clarke, M., & Budimirovic, D.B. (2008). Autism in Genetic Intellectual Disability: Insights into Idiopathic Autism. In Zimmerman, A.W. (Ed.), Autism: Current Theories and Evidence (81-108). Totowa, NJ: The Humana Press Inc.
Kaufmann, W. E., Kidd, S. A., Andrews, H. F., Budimirovic, D. B., Esler, A., Haas-Givler, B., Stackhouse, T., Riley, C., Peacock, G., Sherman, S. L., Brown, W. T., & Berry-Kravis, E. (2017). Autism Spectrum Disorder in Fragile X Syndrome: Cooccurring Conditions and Current Treatment. Pediatrics, 139(Suppl 3), S194–S206.
Kidd, S.A., Berry-Kravis, E., Choo, T.H., Chen, C., Esler, A., Hoffmann, A., Andrews, H.F., & Kaufmann, W.E.. Improving the Diagnosis of Autism Spectrum Disorder in Fragile X Syndrome by Adapting the Social Communication Questionnaire and the Social Responsiveness Scale-2. J Autism Dev Disord. 2020 Sep;50(9):3276-3295.
King, B. H., Veenstra-Vanderweele, J., & Lord, C. (2013). DSM-5 and autism: kicking the tires and making the grade. Journal of the American Academy of Child and Adolescent Psychiatry, 52(5), 454-457. doi: 10.1016/j.jaac.2013.02.009
Landrigan, P.J. (2010) What causes autism? Exploring the environmental contribution. Curr Opin Pediatr, Apr 22(2), 219-25. doi: 10.1097/MOP.0b013e328336eb9a.
Loesch, D.Z., Huggins, R.M., Bui, Q.M., Taylor, A.K., Pratt. C., Epstein, J., & Hagerman, R.J. (2003). Effect of fragile X status categories and FMRP deficits on cognitive profiles estimated by robust pedigree analysis. Am J Med Genet, 122A, 13–23
Lord, C., Petkova, E., Hus, V., Gan, W., Lu, F., & Martin, D. (2011 ). A multisite study of the clinical diagnosis of different autism spectrum disorders. Archives of General Psychiatry, 69, 306-313.
Lord, C., Brugha, T. S., Charman, T., Cusack, J., Dumas, G., Frazier, T., … & Taylor, J. L. (2020). Autism spectrum disorder. Nature reviews Disease primers, 6(1), 1-23.
Macedoni-Luksic, M., Greiss-Hess, L., Rogers, S.J., Gosar, D., Lemons-Chitwood, K., Hagerman, R.J. Imitation in fragile X syndrome – Implications for autism. SAGE Publications and The National Autistic Society Vol 13(6) 599–611; 337850 1362-3613(2009).
Macintosh, K. E., & Dissanayake, C. (2004). Annotation: The similarities and differences between autistic disorder and Asperger’s disorder: a review of the empirical evidence. Journal of Child Psychology and Psychiatry and Allied Disciplines, 45(3), 421-434.
Marschik P.B., Bartl-Pokorny, K.D., Sigafoos, J., Urlesberger, L., Pokorny, F., Didden, R., Einspieler, C., & Kaufmann, W.E. Development of socio-communicative skills in 9- to 12-month-old individuals with fragile X syndrome. Research in Developmental Disabilities 35 (2014) 597–602
Matson, J. L., & Neal, D. (2010). Differentiating communication disorders and autism in children. Research in Autism Spectrum Disorders, 4(4), 626-632.
Mayes, S. D., Black, A, & Tierney, C. D. (2013). DSM-5 under-identifies PDDNOS: Diagnostic agreement between the DSM-5, DSM-IV, and Checklist for Autism Spectrum Disorder. Research in Autism Spectrum Disorders, 7(2), 298-306.
Mazefsky, C. A., McPartland, J. C., Gastgeb, H. Z., & Minshew, N. J. (2013). Brief report: comparability of DSM-IV and DSM-5 ASD research samples. Journal of Autism & Developmental Disorders, 43(5), 1236-1242.
McDuffie, A., Kover, S., Abbeduto, L., Lewis, P., & Brown, T. (2012). Profiles of receptive and expressive language abilities in boys with comorbid fragile X syndrome and autism. American Journal on Intellectual and Developmental Disabilities, 117(1), 18-32.
McPartland, J. C., Reichow, B., & Volkmar, F. R. (2012). Sensitivity and specificity of proposed DSM-5 diagnostic criteria for autism spectrum disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 51(4), 368-383.
Monlux, K.D., Pollard, J.S., Bujanda Rodriguez, A.Y., & Hall, S.S. Telehealth Delivery of Function-Based Behavioral Treatment for Problem Behaviors Exhibited by Boys with Fragile X Syndrome. J Autism Dev Disord. 2019 Jun;49(6):2461-2475.
Muhle, R., Trentacoste S.V., & Rapin, I (2004). The genetics of autism. Pediatrics, 113, 472–486.
Nelson, C. A., Bloom, F. E., Cameron, J. L., Amaral, D., Dahl, R. E., & Pine, D. (2002). An integrative, multidisciplinary approach to the study of brain–behavior relations in the context of typical and atypical development. Development and psychopathology, 14(3), 499-520.
Niu, M., Han, Y., Dy, A., Du, J., Jin, H., Qin, J., Zhang, J., Li, Q., & Hagerman, R.J., (2017) Autism Symptoms in Fragile X Syndrome. Journal of Child Neurology 2017, Vol. 32(10) 903-909
Ozonoff, S., South, M., & Miller, J. (2000). DSM-IV-defined Asperger syndrome: cognitive, behavioral and early history differentiation from high-functioning autism. Autism, 4, 29-46.
Ozonoff, S., Goodlin-Jones, B. L., & Solomon, M. (2005). Evidence-based assessment of autism spectrum disorders in children and adolescents. Journal of Clinical Child and Adolescent Psychology: the official journal for the Society of Clinical Child and Adolescent Psychology, American Psychological Association, Division 53, 34(3), 523-540.
Ozonoff, S. (2012). DSM-5 and autism spectrum disorders–two decades of perspectives from the JCPP. Journal of Child Psychology and Psychiatry and Allied Disciplines, 53(9), e4-e6.
Ozonoff, S., Young, G. S., Carter, A., Messinger, D., Yirmiya, N., Zwaigenbaum, L., … & Hutman, T. (2011). Recurrence risk for autism spectrum disorders: a Baby Siblings Research Consortium study. Pediatrics, 128(3), e488-e495.
Prior, M., Elsenmajer, R., Leekam, S., Wing, L., Gould, J., Ong, B., & Dowe, D. (1998). Are there subgroups within the autistic spectrum? A cluster analysis of a group of children with autistic spectrum disorders. Journal of Child Psychology and Psychiatry and Allied Disciplines, 39(6), 893-902.
Sandbank, M., Bottema-Beutel, K., & Woynaroski, T. Intervention recommendations for children with autism in light of a changing evidence base. JAMA Pediatr. Published online November 09, 2020. doi:10.1001/jamapediatrics.2020.4730
Schaefer, G.B., & Mendelsohn, N.J. (2008). Genetics evaluation for the etiologic diagnosis of autism spectrum disorders. Genet Med, 10, 4–12.
Sidorov, M.S., Auerbach, B.D., & Bear, M.F. (2013) Fragile X mental retardation protein and synaptic plasticity. Mol Brain. Apr 8, 6-15. doi: 10.1186/1756-6606-6-15.
Simacek, J., Dimian, A. F., & McComas, J. J. (2017). Communication intervention for young children with severe neurodevelopmental disabilities via telehealth. Journal of autism and developmental disorders, 47(3), 744-767.
Snow, A. V., & Lecavalier, L. (2011). Comparing autism, PDD-NOS, and other developmental disabilities on parent-reported behavior problems: little evidence for ASD subtype validity. Journal of Autism and Developmental Disorders, 41, 302-310.
Scharfenaker, S., Stackhouse, T., (May 2006. Revised August 2015) Adapting Autism Interventions for Fragile X Syndrome. fragilex.org
Stackhouse, T. (August 2020) Traditional Prompt Hierarchy and modifications for those with FXS. developmentalfx.org
Szatmari, P., Bryson, S. E., Boyle, M. H., Streiner, D. L., & Duku, E. (2003). Predictors of outcome among high functioning children with autism and Asperger syndrome. Journal of Child Psychology and Psychiatry and Allied Disciplines, 44(4), 520-528.
Szatmari, P., Bryson, S., Duku, E., Vaccarella, L., Zwaigenbaum, L., Bennett, T., & Boyle, M.H. (2009). Similar developmental trajectories in autism and Asperger syndrome: from early childhood to adolescence. Journal of Child Psychology and Psychiatry and Allied Disciplines, 50(12), 1459-1467.
Taheri, A., & Perry, A. (2012). Exploring the proposed DSM-5 criteria in a clinical sample. Journal of Autism and Developmental Disorders, 42(9), 1810-1817.
Unholz‑Bowden, E., McComas, J. J., McMaster, K. L., Girtler, S. N., Kolb, R. L., & Shipchandler, A. (2020). Caregiver training via telehealth on behavioral procedures: A systematic review. Journal of Behavioral Education, 29(2), 246-281.
Watson, C., Hoeft, F., Garrett, A.S., Hall, S.S., & Reiss, A.L. (2008). Aberrant Brain Activation During Gaze Processing in Boys with Fragile X Syndrome. Arch Gen Psychiatry, 65(11), 1315-1323. doi:10.1001/archpsyc.65.11.1315.
Weismer, S. E., Lord, C., & Esler, A. (2010). Early language patterns of toddlers on the autism spectrum compared to toddlers with developmental delay. Journal of autism and developmental disorders, 40(10), 1259-1273.
Weitlauf, A. S., Gotham, K. O., Vehorn, A. C., & Warren, Z. E. (2013). Brief Report: DSM-5 “Levels of Support:” A Comment on Discrepant Conceptualizations of Severity in ASD. Journal of Autism and Developmental Disorders, 1-6.
Williams, K., Tuck, M., Helmer, M., Bartak, L., Mellis, C., & Peat, J. K. (2008). Diagnostic labelling of autism spectrum disorders in NSW. Journal of Paediatrics and Child Health, 44(3), 108-113.
Yuen, R. K., Merico, D., Bookman, M., Howe, J. L., Thiruvahindrapuram, B., Patel, R. V., … & Pellecchia, G. (2017). Whole genome sequencing resource identifies 18 new candidate genes for autism spectrum disorder. Nature neuroscience, 20(4), 602-611.
The Fragile X Clinical & Research Consortium was founded in 2006 and exists to improve the delivery of clinical services to families impacted by any Fragile X-associated Disorder and to develop a research infrastructure for advancing the development and implementation of new and improved treatments. Please contact the National Fragile X Foundation for more information: (800) 688-8765

