Zynerba Pharmaceuticals Presents During the 2022 Industry Updates Keynote at the 18th International Fragile X Conference
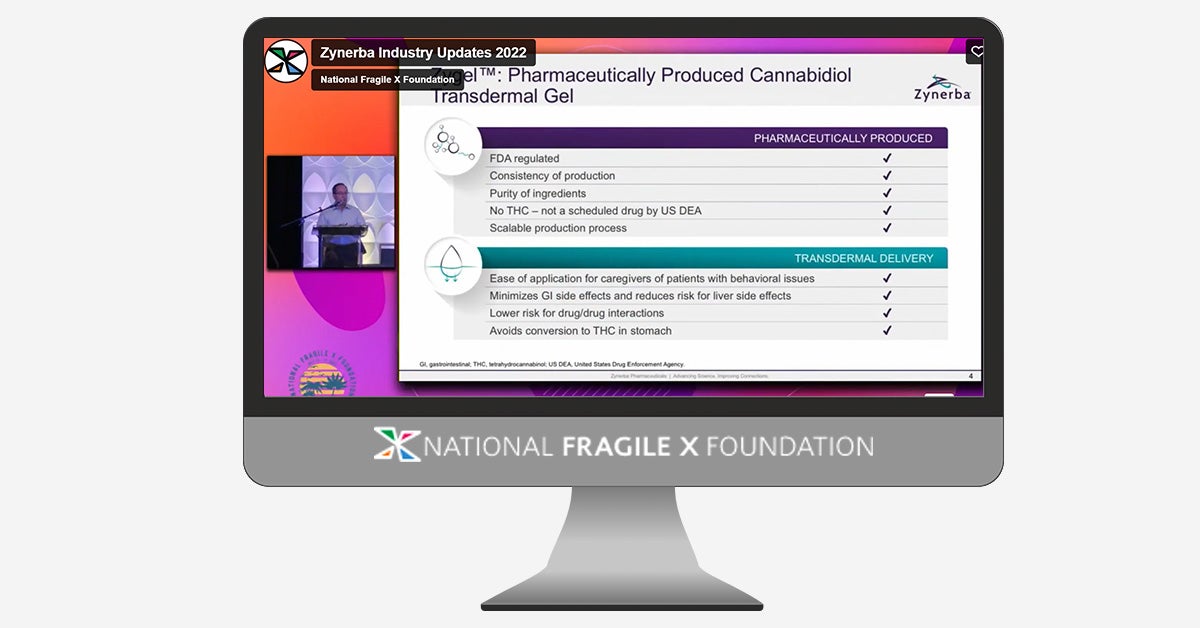
Stephen O’Quinn, Vice President Medical Affairs at Zynerba Pharmaceuticals, presented about ZYN002, a clear, non-plant derived, cannabidiol gel, during the Industry Updates keynote session at the 18th International Fragile X Conference. ZYN002 is in Phase 3 trials in Fragile X Syndrome with the goal of becoming an FDA-approved treatment for the behavioral symptoms of FXS. Zynerba is now enrolling participants in their RECONNECT trial. More information about the RECONNECT trial can be found here.
Stephen spoke about the status Zynerba’s Fragile X program, including the results of their CONNECT-FX trial and their current RECONNECT trial, where they hope to go, and the value of partnering with families with Fragile X. Learn more about Zynerba and ZYN002 by watching their 2022 Industry Updates presentation or vising their MyFXResearch post.