Genetic Testing for Fragile X Syndrome and Associated Disorders
Fragile X testing, analysis, and diagnosis is a complicated subject. This page has extensive information for both parents and medical providers. If you are a parent who is researching this, we know it can be overwhelming, so here are five things you need to know:
- Work with your physician and a licensed genetic counselor. You will need a doctor’s order to have the test done.
- It’s important to test the child, and, if positive, biological family members.
- The cost for the DNA test for Fragile X can vary widely depending on your insurance coverage, deductibles, state you live in, etc. Because of the many variables – the DNA test for Fragile X can range from under $100 up to $1000. However, check with your insurance company for costs and any requirements that need to be met. Note that the test may be covered by insurance, including Medicaid, and may be free or subject to copays/deductibles.
- The test names can vary, but are typically referred to as “Fragile X CGG repeat analysis” or “Fragile X DNA test.”
- The current CPT code, used for billing, is 81243 and may also include 81244.
If you have more questions, we can help. Just email treatment@fragilex.org or call (800) 688-8765.
Note: An important goal of the NFXF is to provide general education about the inheritance pattern and features of Fragile X-associated disorders. The NFXF does not intend this information to serve as medical advice. Individuals and families living with Fragile X-associated disorders should discuss their specific situations — including questions about laboratory results, symptoms, and treatments — with qualified healthcare providers. Given the complexity of Fragile X genetics, it is highly recommended that individuals consult with a genetic counselor or medical geneticist for specific guidance about laboratory testing and results, including the meaning of these findings for family members. For help locating a genetic counselor visit a Fragile X clinic or see Find a Genetic Counselor↗ from the National Society of Genetic Counselors↗.
Who Should Have Fragile X Testing?
There are three general circumstances in which Fragile X testing should be considered:
- Clinical symptoms that suggest Fragile X syndrome, Fragile X-associated tremor/ataxia syndrome (FXTAS), or Fragile X-associated primary ovarian insufficiency (FXPOI).
- A family history of FXS, FXTAS, intellectual or learning disabilities or autism of unknown cause, or infertility.
- Family or personal history of a Fragile X genetics and inheritance (i.e., carrier).
Specific indications for testing include:
- Any male or female with intellectual disabilities, developmental delay, speech and language delay, autism, or learning disabilities of unknown cause.
- Any female with infertility, elevated FSH (follicle-stimulating hormone) levels, premature ovarian failure, primary ovarian insufficiency, or irregular menses.
- Any adult over 50 with features of FXTAS, including intention tremors, ataxia, memory loss, cognitive decline, or personality change, especially in combination with a positive family history of Fragile X.
- Any preconception or pregnant woman who expresses interest in or requests Fragile X carrier testing.
Genetic Testing
Genetic testing is a clinical diagnostic tool often used to search for the underlying cause of a child’s developmental delays, autism, or intellectual disability.
The first diagnostic genetic test for Fragile X syndrome (FXS) involved looking at the X chromosome under a microscope. In the 1970s, it was observed that some males with inherited intellectual disability had an X chromosome that appeared “fragile,” as if the end had broken off. This is actually where the name “Fragile X” originated. This testing was fairly accurate for identifying males with Fragile X syndrome but could not reliably identify females with Fragile X syndrome or premutation carriers.
In the 1990s, genetic testing technologies improved, and the specific gene associated with Fragile X syndrome — FMR1 — was discovered. Since then, highly accurate Fragile X DNA testing has been widely available to identify individuals with all types of repeat expansions within the FMR1 gene.
Recommended genetic testing includes:
- Chromosomal microarray analysis, which looks for extra or missing pieces of genetic material.
- Exome sequencing, which reads through part of a person’s genetic code.
- A specific DNA test for Fragile X syndrome.
Currently, Fragile X testing must be ordered as a separate test since expansions of the FMR1 gene cannot be detected through microarray or exome sequencing.
Two main testing techniques are used when diagnosing Fragile X-associated disorders:
- Polymerase chain reaction (PCR): This technique is able to identify the size of the repetitive section of the FMR1 gene, including CGG repeat numbers in the normal, intermediate, premutation, and full mutation ranges.
- Southern blot analysis: For full mutations, labs generally follow up with a Southern blot analysis to confirm whether the gene is methylated, a chemical change that prevents it from producing its normal protein, known as FMRP.
Testing for AGG interruptions can be done as a separate test and is most relevant as part of carrier testing. AGG analysis is often ordered as a follow-up test when a premutation or intermediate result is reported in women carriers who plan to have children.
Laboratories typically report the number of CGG repeats within the FMR1 gene. For full mutations, they also report the methylation status and the presence of mosaicism if it’s detected. Because Fragile X testing is widely available through many different laboratories, not all lab reports look the same. Additionally, many primary care providers and non-genetics specialists are unfamiliar with interpreting Fragile X lab reports.
Given the complexity of Fragile X inheritance, it is very important that families living with Fragile X-associated disorders meet with a genetic counselor or geneticist to review the meaning of a positive lab report for the individual and his or her extended family.
The Fragile X Test and Lab Report
What’s the name of the test I should ask for?
Each laboratory may name its specific test differently, but the description will typically refer to “Fragile X CGG repeat analysis” or the “Fragile X DNA test.” This test is used to diagnose Fragile X syndrome and to determine whether someone (male or female) is a premutation carrier, which is used for determining FXPOI (Fragile X-associated primary ovarian insufficiency) and FXTAS (Fragile X-associated tremor/ataxia syndrome).
The test name may mention “PCR with reflex to Southern blot,” meaning that if a full mutation is detected by PCR (polymerase chain reaction), the methylation status will automatically be checked using Southern blot.
Fragile X DNA testing orders usually include a CPT number, which is a procedure code used for billing purposes. The current CPT code for Fragile X testing is 81243, sometimes including 81244 at some laboratories. If a healthcare provider is unsure about how to order a Fragile X DNA test, they can contact the lab’s customer service number for assistance.
What’s included in a Fragile X laboratory report?
The majority of clinical laboratories report the CGG repeat number, as well as the methylation status if a full mutation is detected. Labs also indicate mosaicism if present.
What about AGG numbers, mosaicism, methylation, and FMR1 protein levels?
The number of AGG interruptions↗ has no impact on a person’s Fragile X syndrome symptoms and is not routinely analyzed or reported when testing is done to evaluate the cause of developmental delays. It is sometimes reported on those with the premutation. Families should discuss the lab report with a genetic counselor and can ask questions at that time about any information they do not see on the report.
Clinical laboratories do not offer testing to measure Fragile X protein (FMRP) levels, since this information is not useful for medical care at the present time. For specific questions, ask your genetic counselor or other qualified healthcare provider to review the lab report with you.
Requesting Fragile X Testing
Do I need a doctor’s order to get Fragile X testing?
Yes. Major genetic testing labs currently require an order from a healthcare provider. This is an important safeguard to ensure that individuals being tested receive appropriate notification, education, follow-up, and medical support about the meaning of the results.
How long does it take to get the results?
The amount of time varies by lab, but results should be expected within two to four weeks.
Cost and Insurance Coverage
How much does Fragile X testing cost, and does insurance cover the testing?
The cost of Fragile X DNA testing can range from under $100 up to $1000 (U.S. dollars).
Many factors influence insurance coverage and out-of-pocket costs related to genetic testing. In the U.S., many individuals with Medicaid as a primary or secondary insurance may not be responsible for any out-of-pocket costs. For those with private insurance, coverage varies depending on the company, and a family’s cost depends on a number of different factors.
While some insurers do not cover any genetic testing, most offer it as a covered benefit under certain circumstances, such as the diagnostic evaluation of a child with developmental delay. If genetic testing is a covered benefit, families may still be responsible for some costs, depending on whether they have met their deductible or if their specific policy requires a co-pay or co-insurance.
The genetic testing laboratory or ordering healthcare provider may have office staff who can assist families in determining their estimated out-of-pocket cost for Fragile X testing.
If there are any questions before proceeding, it’s a good idea for families to call their insurance company to find out specifics, including the remaining deductible and the expected out-of-pocket cost for a Fragile X DNA test.
Test Samples
Will I need to submit a blood or a saliva sample? Which is more accurate?
In addition to blood samples, many labs now offer the ability to do Fragile X testing on a saliva or buccal (cheek swab) sample.
The testing is very accurate for all sample types, and the type of sample submitted does not influence the results. The same type of test is used regardless of whether DNA is extracted from blood, saliva, or cheek cells.
Occasionally, the lab is not able to get enough DNA from a saliva or buccal sample and may ask for a repeat sample. This happens far less often with blood samples.
A laboratory’s website will often state the types of samples they accept for Fragile X DNA testing, and a healthcare provider can also call the laboratory to verify this information.
CGG Repeats
Where do I find my CGG repeat numbers and what do they mean?
Laboratory reports typically include the number of CGG repeats detected through PCR analysis, based on the following interpretation standard:
- Normal: less than 45 CGG repeats
- Intermediate: 45-54 CGG repeats
- Premutation: 55-200 CGG repeats
- Full mutation: greater than 200 CGG repeats
If you have questions or can’t find the number of repeats listed, you should consult with a genetic counselor or other qualified healthcare professional.
What Do My Test Results Mean?
Also see our short 2-minute video below this list:
- If you have results in the normal (i.e., typical) range, you are not at risk to have a child with Fragile X syndrome.
- If you have an intermediate (i.e., “gray zone”) result, you are not at risk to have a child with Fragile X syndrome. However, in future generations of your family, the CGG repeat may expand to the premutation range.
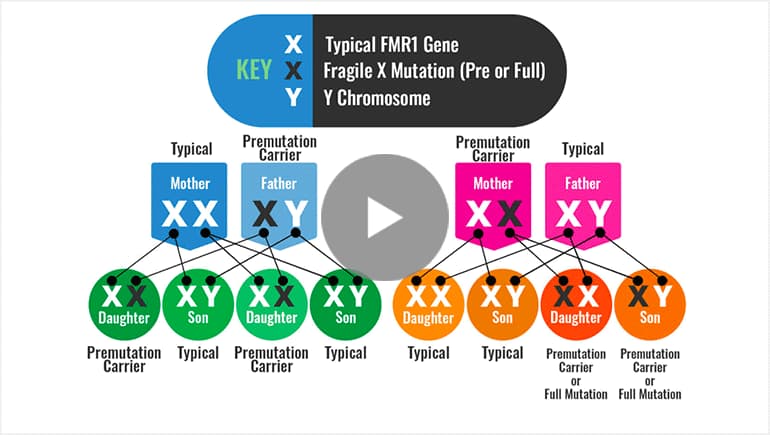
- If you have a premutation and you are male, all of your daughters will be premutation carriers. Your sons will receive your Y chromosome and will not be premutation carriers. You may be at risk to develop FXTAS.
- If you have a premutation and are female, you are at risk to have a child with FXS and are also at risk for infertility and early menopause (FXPOI), and to a lesser extent, FXTAS.
- If you have a full mutation and you are female, you may have physical or learning issues related to Fragile X syndrome. You are also at risk to have a child with Fragile X syndrome.
- If your son has a full mutation, he will likely exhibit some level of intellectual disability, including language or behavioral issues that will benefit from professional services, including special education.
- If your daughter has a full mutation, she is at risk for Fragile X syndrome or may exhibit only mild or no features of the condition.
Watch — How the Fragile X Premutation or Full Mutation is Passed On
Methylation
What is methylation and is it included when you get tested?
When a full mutation is present in the FMR1 gene, a chemical reaction called methylation typically occurs and turns off the FMR1 gene, causing FXS. Think of methylation like a light switch for a gene.
- If there is no methylation, then the gene is “turned on” and functioning in the body.
- If methylation is present, then the gene is “turned off” and cannot function.
Most labs report methylation status when a Fragile X full mutation is detected, but this is not typically included on reports for premutation or intermediate expansions.
Families who have questions about methylation should discuss the specifics with a genetic counselor.
Mosaicism
What is mosaic or mosaicism and is it included in the report?
You might hear a parent say their child is mosaic and have no idea if this applies to your child. In genetics, mosaicism means that there are two or more genetically different sets of cells in a person’s body.
For Fragile X, there can be mosaicism for different sizes of CGG repeats or different levels of methylation. Most testing laboratories are able to detect significant mosaicism as part of Fragile X DNA testing but will only note it on the lab report if the sample is positive.
If a report makes no mention of it, then the lab most likely did not find mosaicism. In any case, it’s important that you review your child’s Fragile X lab report with a genetic counselor or other qualified healthcare professional who can best interpret the meaning of the results.
FMR1 Protein Levels
Do the labs ever test how much FMR1 protein is being produced? Is it important?
The FMR1 gene provides instructions for making a protein called FMRP. Currently, clinical laboratories do not offer testing for Fragile X protein levels, although this testing is sometimes done as part of research projects.
At this time, information about FMRP levels cannot predict a child’s future functioning or be used medically to inform treatment options, so it is not considered a clinically useful test. That being said, the study of FMRP on a research basis may one day advance our understanding of Fragile X-associated disorders.
AGG Interruptions
Do labs ever test for AGG interruptions, and what would this tell us?
There is no evidence that the number of AGG interruptions↗ has an impact a person’s Fragile X syndrome symptoms, and it’s not usually analyzed or included in test results from children with FXS.
AGG testing is most informative for family planning, since it can help couples estimate the chance that an FMR1 gene might expand when inherited.
AGG interruptions serve to stabilize an FMR1 premutation or intermediate allele to keep it from expanding when passed down from a parent to a child at conception. A person can have 0, 1, 2, or 3 AGG interruptions in their FMR1 gene. For small premutations and intermediate alleles, the more AGGs present, the less likely the FMR1 gene will expand. However, for premutations with ~90 CGG repeats or more, the number of AGGs is not informative, since it does not significantly change the estimated chance of expansion to a full mutation in the next generation.
Females and Carrier Status
Should the female children of male Fragile X carriers be tested?
Based on our understanding of Fragile X inheritance, a father with an FMR1 premutation will pass it on to all of his daughters and none of his sons. Premutations are very unlikely to expand to full mutations when inherited from a father, and even in the absence of testing, his daughters are all presumed to be premutation carriers. A daughter’s actual premutation repeat number could be a bit larger, or even smaller, than her father’s.
Fragile X testing should not be done on asymptomatic young girls simply to confirm their carrier status. A decision about carrier testing should be made by the girl herself once she is old enough to make an informed choice.
A woman’s decision whether or not to be tested is up to her and will depend on her particular situation. Some women understand that they are carriers based on a father’s testing but have little interest in being tested themselves. Other women may want to confirm their exact number of repeats, including the number of AGG interruptions, for family planning or other reasons.
If I don’t plan on having more children, do I need to know my carrier status?
Say your son has Fragile X syndrome, and it was determined through family history that you were the carrier, and it came from your father. You’ve never been tested to find out exactly what your carrier status is, but are not planning on having any more children. Should you?
The decision about whether or not to pursue genetic testing is a deeply personal choice. As a carrier, knowing your exact repeat size is unlikely to change your immediate medical management. Although you presumably inherited a premutation from your father, testing would confirm this, as well as make you aware of your potential to develop FXTAS or FXPOI.
Final Note
Given the complexity of Fragile X inheritance, it is very important that families living with Fragile X-associated disorders meet with a genetic counselor or geneticist to review the meaning of a positive lab report for the individual and his or her extended family.
For help locating a genetic counselor visit a Fragile X clinic or see Find a Genetic Counselor↗ from the National Society of Genetic Counselors↗.
LEARN MORE
Understanding the Fragile X Syndrome Diagnosis and Associated Developmental Diagnoses
We cut through the confusion about the meaning of Fragile X syndrome in relation to other types of diagnoses that a child receives.
Fragile X Genetics & Inheritance
Even for genetics professionals, FMR1 inheritance is complex and confusing, so it’s no surprise that families often have questions about the genetics of Fragile X.
Fragile X Syndrome & Autism
When associated with FXS, autism is caused by the genetic change or mutation in the Fragile X gene — the most common genetic cause of autism.
A Blood Test for Autism? Not so Fast
The notion that autism itself can be directly diagnosed through a blood test is incorrect and misleading.
The Who, What & How for Genetic Counseling
Who should seek counseling? What does a genetic counselor do? How do I find one?