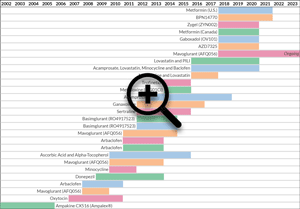
Past and Present Clinical Trials in Fragile X Syndrome
There have been quite a few clinical trials in Fragile X syndrome. We provide the table below to document current and past clinical trials and will keep it updated as we learn of new trials.
How Clinical Trials Work: Learn more about clinical trials, including what they are, how they work, and how you can participate.
Search MyFXResearch Portal: Search for currently available clinical trial and study opportunities.
All columns are sortable. Click on the column head titles (END YR, START YR, PHASE, etc.) to change A–Z to Z–A (or 1–10 and 10–1). The default sort shows from most to least recent. To sort the full list, you might want to first choose to show all, then sort your columns.
There are multiple pages. You can advance from page to page using the links at the bottom right of the table. You can also choose to show more or all rows (10, 25, 50, 100) without pagination by using the Show ___ entries dropdown at the top left of the table.
The plus sign — ![]() — provides more information. Click on the plus sign to the left of any row to see more about that clinical trial: Measure (primary outcome measure), sponsor, trial size, a link to each ClinicalTrials.gov identifier, and, once completed, any results that are available.
— provides more information. Click on the plus sign to the left of any row to see more about that clinical trial: Measure (primary outcome measure), sponsor, trial size, a link to each ClinicalTrials.gov identifier, and, once completed, any results that are available.
We have footnotes and a glossary to help you understand abbreviations and terms. If you’re unfamiliar with any of the abbreviations or terminology — see the footnotes and glossary at the end of this page. Or contact us for additional help.
You can search for any keyword. Use the Search box at the top right of the table to search, for example, a drug name, sponsor name, or indication terms (memory, behavior, etc.)
Need help? Please contact Hilary Rosselot with your questions.
ClinicalTrials.gov↗
A database of privately and publicly funded clinical studies. Also, see NCT Link.
EEG
A recording of electrical brain activity (brain waves) made by an electroencephalograph (EEG).
Indication
The symptom indicates the need for a certain medical treatment.
NCT Link
This is the ClinicalTrials.gov unique identifier, also referred to as “NCT number↗,” assigned to each clinical study record. Example: NCT04130360.
Measure
This is the abbreviated version we use for “primary outcome measure,” which is the main outcome measure↗ the clinical study has identified to determine the intervention or treatment’s effectiveness. Many also have secondary outcome measures, which can be found using the NCT link.
Phase
The current phase↗, or stage, of a clinical trial. Also see our article How Clinical Trials Work: From Start to Finish.
Primary Outcome Measure
See Measure.
ABC-C — Aberrant Behavior Checklist-Community
ABC-Cfx — Aberrant Behavior Checklist-Community, Fragile X Factor Structure
AE — Adverse Events
ADAMS — Anxiety Depression and Mood Scale
ADHD-RS — Attention Deficit Hyperactivity Disorder – Rating Scale
APP — Amyloid Precursor Protein
CGI — Clinical Global Impression
CNT — Contingency Naming Test
Conners — Conners’ Rating Scales
ELS — Expressive Language Sampling
MSEL — Mullen Scales of Early Learning
NDW — Number of Different Words
PILI — Parent-Implemented Language Intervention
SAE — Serious Adverse Events
TEAE — Treatment Emergent Adverse Events
VAS — Visual Analogue Scale
Past and Present Clinical Trials in Fragile X Syndrome
| End Yr: | Start Yr: | Drug: | Phase: | Primary Outcome Measure: | Gender: | Age: | Sponsor: | Trial Size: | NCT Link: | Notes: | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2013 | 2009 | Donepezil | 2 | Executive functioning | All | 12-29 | Stanford University | 45 | NCT01120626 |
||
| 2020 | 2018 | Zygel (ZYN002) | 2/3 | Fragile X behaviors – ABC-Cfx | All | 3-17 | Zynerba Pharmaceuticals | 212 | NCT03614663 |
Full drug name: Zygel (ZYN002) – CBD Transdermal Gel |
|
| 2015 | 2014 | Trofinetide | 2 | Safety (secondary included physiological, behavior, and global/ functional assessments) | Males | 12-45 | Neuren Pharmaceuticals | 72 | NCT01894958 |
||
| 2015 | 2012 | Sertraline | 2 | Cognition, language – Mullen and CGI | All | 2.5-5 | UC Davis | 57 | NCT01474746 |
||
| 2017 | 2016 | Minocycline and Lovastatin | 2 | Fragile X behaviors | All | 13-40 | Université de Sherbrooke | 23 | NCT02680379 |
Results: Drug duo delivers brain, behavioral benefits for fragile X syndrome |
|
| 2011 | 2010 | Minocycline | n/a | Clinical impression – CGI and VAS behavior | All | 3.5-16 | UC Davis | 66 | NCT01053156 |
||
| 2010 | 2007 | Oxytocin | 2 | Fragile X behaviors – Eye contact challenge task | Males | 13-29 | Stanford University | 10 | NCT01254045 |
Results: Effects of intranasal oxytocin on social anxiety in males with fragile X syndrome |
|
| 2021 | 2018 | Metformin (U.S.) | 2/3 | Fragile X symptoms (language, behavior, weight) – ELS mean NDW score | All | 6-25 | UC Davis | 55 | NCT03479476 |
Results: Metformin treatment in young children with fragile X syndrome |
|
| 2020 | 2018 | Metformin (Canada) | 2 | Safety (secondary was behavior and cognition) – AE and ABC-C | All | 10-45 | Université de Sherbrooke | 15 | NCT03722290 |
Results: Metformin treatment in young children with fragile X syndrome |
|
| 2015 | 2014 | Metadoxine (MG01CI) | 2 | Inattention – ADHD RS | All | 15-55 | Alcobra | 62 | NCT02126995 |
Results: Alcobra Announces Results From Phase 2 Clinical Trial of MDX for Fragile X Syndrome |
|
| 2009 | 2008 | Mavoglurant (AFQ056) | 2 | Fragile X behaviors – ABC-Cfx | Males | 18-35 | Novartis | 30 | NCT00718341 |
||
| 2023 | 2017 | AFQ056 (Mavoglurant) and Language Intervention | 2 | Language – Weighted child intentional communication score | All | 2.5-6 | Elizabeth Berry-Kravis, Rush University Medical Center | 99 | NCT02920892 |
This trial is complete, the results will be published soon. |
|
| 2013 | 2010 | Mavoglurant (AFQ056) | 2 | Fragile X behaviors – ABC-Cfx | All | 18-45 | Novartis | 175 | NCT01253629 |
||
| 2020 | 2016 | Lovastatin and PILI | 4 | Fragile X behaviors, language – ELS | All | 10-17 | UC Davis | 30 | NCT02642653 |
||
| 2016 | 2012 | Ganaxolone | 2 | Clinical impression – CGI | All | 6-17 | Marinus Pharmaceuticals | 59 | NCT01725152 |
||
| 2020 | 2018 | Gaboxadol (OV101) | 2 | Safety – Incidence of AEs (secondary was ABC-C and CGI-I) | Males | 13-22 | Ovid Therapeutics | 23 | NCT03697161 |
Results: Gaboxadol in Fragile X Syndrome: A 12-Week Randomized, Double-Blind, Parallel-Group, Phase 2a Study |
|
| 2014 | 2011 | Mavoglurant (AFQ056) | 2 | Fragile X behaviors – ABC-Cfx | All | 12-17 | Novartis | 139 | NCT01357239 |
||
| 2021 | 2018 | BPN14770 | 2 | Safety, tolerability – Treatment emergent AE (TEAE) | Males | 18-45 | Tetra Discovery Partners | 30 | NCT03569631 |
||
| 2014 | 2012 | Basimglurant (RO4917523) | 2 | Safety | All | 5-13 | Hoffmann-La Roche | 47 | NCT01750957 |
Results: From a presentation of the International Society of Autism Research (ISAR): Safety and Exploratory Efficacy of Basimglurant in Pediatric Patients with Fragile X Syndrome: A Randomized, Double-Blind, Placebo-Controlled Study |
|
| 2014 | 2012 | Basimglurant (RO4917523) | 2 | Anxiety, depression – ADAMS | All | 14-50 | Roche | 185 | NCT01517698 |
||
| 2020 | 2018 | AZD7325 | 1 | Safety – APP | All | 18-50 | CCHMC | 15 | NCT03140813 |
n/a |
|
| 2015 | 2010 | Ascorbic Acid and Alpha-Tocopherol | 2 | Hyperactivity – Conners | Males | 6-18 | The Mediterranean Institute for the Advance of Biotechnology and Health Research | 30 | NCT01329770 |
Ascorbic acid is Vitamin C and alpha-tocopherol is Vitamin E. |
|
| 2010 | 2008 | Arbaclofen | 2 | Irritability – ABC-C | All | 6-40 | Seaside Therapeutics | 45 | NCT01013480 |
||
| 2013 | 2011 | Arbaclofen | 3 | Lethargy, social withdrawal – ABC-C | All | 5-11 | Seaside Therapeutics | 172 | NCT01325220 |
||
| 2013 | 2011 | Arbaclofen | 3 | Social avoidance – ABC-C | All | 12-50 | Seaside Therapeutics | 125 | NCT01282268 |
||
| 2005 | 2002 | Ampakine CX516 (Ampalex®) | 2 | Memory – various measures | All | 18-50 | RespireRx | 49 | NCT00054730 |
Results have been submitted, but are not yet available. Also see: Effect of CX516, an AMPA-modulating compound, on cognition and behavior in fragile X syndrome: a controlled trial |
|
| 2020 | 2016 | Acamprosate, Lovastatin, Minocycline and Baclofen | 1 | Brain activity, clinical impression – EEG and CGI-I | All | 15-55 | Children's Hospital Medical Center, Cincinnati | 29 | NCT02998151 |
Results: Neurophysiological and Acute Pharmacological Studies in FXS Patients — Study Results |
|
| 2018 | 2013 | Acamprosate | 1 | Social avoidance – ABC-C | All | 5-23 | Children's Hospital Medical Center, Cincinnati | 46 | NCT01911455 |
n/a |
*Closed for recruiting.
This table has been adapted from the Drug development for neurodevelopmental disorders: lessons learned from fragile X syndrome (2017) publication and updated based on ClinicalTrials.gov.
Berry-Kravis, E. M., Lindemann, L., Jønch, A. E., Apostol, G., Bear, M. F., Carpenter, R. L., Crawley, J. N., Curie, A., Des Portes, V., Hossain, F., Gasparini, F., Gomez-Mancilla, B., Hessl, D., Loth, E., Scharf, S. H., Wang, P. P., Von Raison, F., Hagerman, R., Spooren, W., & Jacquemont, S. (2018). Drug development for neurodevelopmental disorders: lessons learned from fragile X syndrome. Nature reviews. Drug discovery, 17(4), 280–299. https://doi.org/10.1038/nrd.2017.221
This table has been adapted from the Drug development for neurodevelopmental disorders: lessons learned from fragile X syndrome (2017)↗ publication and updated based on ClinicalTrials.gov↗.
Berry-Kravis, E. M., Lindemann, L., Jønch, A. E., Apostol, G., Bear, M. F., Carpenter, R. L., Crawley, J. N., Curie, A., Des Portes, V., Hossain, F., Gasparini, F., Gomez-Mancilla, B., Hessl, D., Loth, E., Scharf, S. H., Wang, P. P., Von Raison, F., Hagerman, R., Spooren, W., & Jacquemont, S. (2018). Drug development for neurodevelopmental disorders: lessons learned from fragile X syndrome. Nature reviews. Drug discovery, 17(4), 280–299. https://doi.org/10.1038/nrd.2017.221
Questions?
If you have questions about anything research-related, we’d love to hear from you! You can reach out to Hilary Rosselot directly, or submit your question or comment through our contact form below.

Hilary Rosselot, Director of Research Facilitation
hilary@fragilex.org | (202) 747-6208
THANK YOU
Image by mohamed Hassan from Pixabay